TAM receptors and the clearance of apoptotic cells
- PMID: 20958312
- PMCID: PMC3061224
- DOI: 10.1111/j.1749-6632.2010.05744.x
TAM receptors and the clearance of apoptotic cells
Abstract
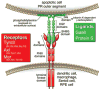
The Tyro3, Axl, and Mer (TAM) receptor tyrosine kinases and their ligands Gas6 and Protein S are required for the optimal phagocytosis of apoptotic cells in the mature immune, nervous, and reproductive systems. Genetic analyses in mice, rats, and humans reveal that this receptor-ligand system plays an especially important role in the phagocytosis that is triggered by the "eat-me" signal phosphatidylserine. Deficiencies in TAM signaling lead to human retinal dystrophies and may contribute to lupus and other human autoimmune diseases. The TAM system appears to interact and cooperate with several other phagocytic networks, including scavenger receptor and integrin-based systems, and may serve as a signaling hub that integrates these systems.
© 2010 New York Academy of Sciences.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell 2011. 2010;40:619–630. - PubMed
-
- Lai C, Lemke G. An extended family of protein-tyrosine kinase genes differentially expressed in the vertebrate nervous system. Neuron. 1991;6:691–704. - PubMed
-
- Manning G, Whyte DB, Martinez R, et al. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

