Disorders of dysregulated signal traffic through the RAS-MAPK pathway: phenotypic spectrum and molecular mechanisms
- PMID: 20958325
- PMCID: PMC3010252
- DOI: 10.1111/j.1749-6632.2010.05790.x
Disorders of dysregulated signal traffic through the RAS-MAPK pathway: phenotypic spectrum and molecular mechanisms
Abstract
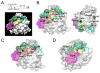
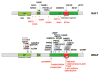
RAS GTPases control a major signaling network implicated in several cellular functions, including cell fate determination, proliferation, survival, differentiation, migration, and senescence. Within this network, signal flow through the RAF-MEK-ERK pathway-the first identified mitogen-associated protein kinase (MAPK) cascade-mediates early and late developmental processes controlling morphology determination, organogenesis, synaptic plasticity, and growth. Signaling through the RAS-MAPK cascade is tightly controlled; and its enhanced activation represents a well-known event in oncogenesis. Unexpectedly, in the past few years, inherited dysregulation of this pathway has been recognized as the cause underlying a group of clinically related disorders sharing facial dysmorphism, cardiac defects, reduced postnatal growth, ectodermal anomalies, variable cognitive deficits, and susceptibility to certain malignancies as major features. These disorders are caused by heterozygosity for mutations in genes encoding RAS proteins, regulators of RAS function, modulators of RAS interaction with effectors, or downstream signal transducers. Here, we provide an overview of the phenotypic spectrum associated with germline mutations perturbing RAS-MAPK signaling, the unpredicted molecular mechanisms converging toward the dysregulation of this signaling cascade, and major genotype-phenotype correlations.
© 2010 New York Academy of Sciences.
Figures



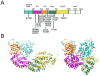

References
-
- Mitin N, Rossman KL, Der CJ. Signaling interplay in Ras superfamily function. Curr Biol. 2005;15:R563–574. - PubMed
-
- Sundaram MV. RTK/Ras/MAPK signaling. WormBook. 2006;11:1–19. - PubMed
-
- McKay MM, Morrison DK. Integrating signals from RTKs to ERK/MAPK. Oncogene. 2007;26:3113–3121. - PubMed
-
- Bos JL. Ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

