Residue-specific side-chain packing determines the backbone dynamics of transmembrane model helices
- PMID: 20959095
- PMCID: PMC2955363
- DOI: 10.1016/j.bpj.2010.08.031
Residue-specific side-chain packing determines the backbone dynamics of transmembrane model helices
Abstract
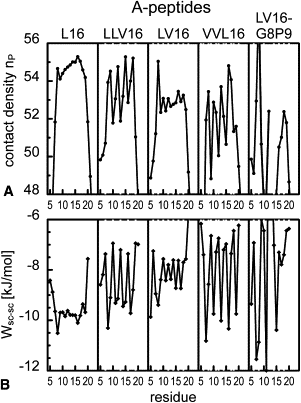
The transmembrane domains (TMDs) of membrane-fusogenic proteins contain an overabundance of β-branched residues. In a previous effort to systematically study the relation among valine content, fusogenicity, and helix dynamics, we developed model TMDs that we termed LV-peptides. The content and position of valine in LV-peptides determine their fusogenicity and backbone dynamics, as shown experimentally. Here, we analyze their conformational dynamics and the underlying molecular forces using molecular-dynamics simulations. Our study reveals that backbone dynamics is correlated with the efficiency of side-chain to side-chain van der Waals packing between consecutive turns of the helix. Leu side chains rapidly interconvert between two rotameric states, thus favoring contacts to its i±3 and i±4 neighbors. Stereochemical restraints acting on valine side chains in the α-helix force both β-substituents into an orientation where i,i±3 interactions are less favorable than i,i±4 interactions, thus inducing a local packing deficiency at VV3 motifs. We provide a quantitative molecular model to explain the relationship among chain connectivity, side-chain mobility, and backbone flexibility. We expect that this mechanism also defines the backbone flexibility of natural TMDs.
Copyright © 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Doig A.J., Errington N., Iqbalsyah T.M. Stability and design of α-helices. In: Buchner J., Kiefhaber T., editors. Handbook of Protein Folding. Wiley, Weinheim; Germany: 2005. pp. 247–313.
-
- Blaber M., Zhang X.J., Matthews B.W. Structural basis of amino acid α helix propensity. Science. 1993;260:1637–1640. - PubMed
-
- Blaber M., Zhang X.J., Matthews B.W. Determination of α-helix propensity within the context of a folded protein. Sites 44 and 131 in bacteriophage T4 lysozyme. J. Mol. Biol. 1994;235:600–624. - PubMed
-
- Chellgren B.W., Creamer T.P. Side-chain entropy effects on protein secondary structure formation. Proteins. 2006;62:411–420. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

