Multicolor fluorescence nanoscopy in fixed and living cells by exciting conventional fluorophores with a single wavelength
- PMID: 20959110
- PMCID: PMC2956215
- DOI: 10.1016/j.bpj.2010.08.012
Multicolor fluorescence nanoscopy in fixed and living cells by exciting conventional fluorophores with a single wavelength
Abstract
Current far-field fluorescence nanoscopes provide subdiffraction resolution by exploiting a mechanism of fluorescence inhibition. This mechanism is implemented such that features closer than the diffraction limit emit separately when simultaneously exposed to excitation light. A basic mechanism for such transient fluorescence inhibition is the depletion of the fluorophore ground state by transferring it (via a triplet) in a dark state, a mechanism which is workable in most standard dyes. Here we show that microscopy based on ground state depletion followed by individual molecule return (GSDIM) can effectively provide multicolor diffraction-unlimited resolution imaging of immunolabeled fixed and SNAP-tag labeled living cells. Implemented with standard labeling techniques, GSDIM is demonstrated to separate up to four different conventional fluorophores using just two detection channels and a single laser line. The method can be expanded to even more colors by choosing optimized dichroic mirrors and selecting marker molecules with negligible inhomogeneous emission broadening.
Copyright © 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



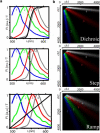
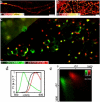
References
-
- Abbe E. Contributions to the theory of the microscope and microscopic perception [Beiträge zur Theorie des Mikroskops und der mikroskopischen Wahrnehmung] Archiv für Mikroskopische Anatomie. 1873;9:413–468.
-
- Hell S.W., Wichmann J. Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 1994;19:780–782. - PubMed
-
- Hell S.W., Jakobs S., Kastrup L. Imaging and writing at the nanoscale with focused visible light through saturable optical transitions. Appl. Phys., A Mater. Sci. Process. 2003;77:859–860.
-
- Hell S.W. Far-field optical nanoscopy. Science. 2007;316:1153–1158. - PubMed
-
- Hell S.W., Kroug M. Ground-state depletion fluorescence microscopy, a concept for breaking the diffraction resolution limit. Appl. Phys. B. 1995;60:495–497.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

