Enhancement of reactive oxygen species production and chlamydial infection by the mitochondrial Nod-like family member NLRX1
- PMID: 20959452
- PMCID: PMC3009891
- DOI: 10.1074/jbc.M110.137885
Enhancement of reactive oxygen species production and chlamydial infection by the mitochondrial Nod-like family member NLRX1
Abstract
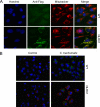
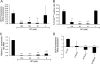
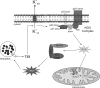
Chlamydia trachomatis infections cause severe and irreversible damage that can lead to infertility and blindness in both males and females. Following infection of epithelial cells, Chlamydia induces production of reactive oxygen species (ROS). Unconventionally, Chlamydiae use ROS to their advantage by activating caspase-1, which contributes to chlamydial growth. NLRX1, a member of the Nod-like receptor family that translocates to the mitochondria, can augment ROS production from the mitochondria following Shigella flexneri infections. However, in general, ROS can also be produced by membrane-bound NADPH oxidases. Given the importance of ROS-induced caspase-1 activation in growth of the chlamydial vacuole, we investigated the sources of ROS production in epithelial cells following infection with C. trachomatis. In this study, we provide evidence that basal levels of ROS are generated during chlamydial infection by NADPH oxidase, but ROS levels, regardless of their source, are enhanced by an NLRX1-dependent mechanism. Significantly, the presence of NLRX1 is required for optimal chlamydial growth.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

