Enterohemorrhagic Escherichia coli induce attaching and effacing lesions and hemorrhagic colitis in human and bovine intestinal xenograft models
- PMID: 20959635
- PMCID: PMC3014348
- DOI: 10.1242/dmm.005777
Enterohemorrhagic Escherichia coli induce attaching and effacing lesions and hemorrhagic colitis in human and bovine intestinal xenograft models
Abstract
Enterohemorrhagic Escherichia coli (EHEC) O157:H7 is an important cause of diarrhea, hemorrhagic colitis and hemolytic uremic syndrome in humans worldwide. The two major virulence determinants of EHEC are the Shiga toxins (Stx) and the type III secretion system (T3SS), including the injected effectors. Lack of a good model system hinders the study of EHEC virulence. Here, we investigated whether bovine and human intestinal xenografts in SCID mice can be useful for studying EHEC and host tissue interactions. Fully developed, germ-free human and bovine small intestine and colon were established by subcutaneous transplantation of human and bovine fetal gut into SCID mice. Xenografts were allowed to develop for 3-4 months and thereafter were infected by direct intraluminal inoculation of Stx-negative derivatives of EHEC O157:H7, strain EDL933. The small intestine and colon xenografts closely mimicked the respective native tissues. Upon infection, EHEC induced formation of typical attaching and effacing lesions and tissue damage that resembled hemorrhagic colitis in colon xenografts. By contrast, xenografts infected with an EHEC mutant deficient in T3SS remained undamaged. Furthermore, EHEC did not attach to or damage the epithelium of small intestinal tissue, and these xenografts remained intact. EHEC damaged the colon in a T3SS-dependent manner, and this model is therefore useful for studying the molecular details of EHEC interactions with live human and bovine intestinal tissue. Furthermore, we demonstrate that Stx and gut microflora are not essential for EHEC virulence in the human gut.
Figures



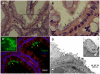

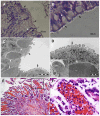

References
-
- Cantey J.R., Inman L.R., Blake R.K. (1989). Production of diarrhea in the rabbit by a mutant of Escherichia coli (RDEC-1) that does not express adherence (AF/R1) pili. J. Infect. Dis. 160, 136–141 - PubMed
-
- Chong Y., Fitzhenry R., Heuschkel R., Torrente F., Frankel G., Phillips A.D. (2007). Human intestinal tissue tropism in Escherichia coli O157: H7-initial colonization of terminal ileum and Peyer’s patches and minimal colonic adhesion ex vivo. Microbiology 153, 794–802 - PubMed
-
- Croxen M.A., Finlay B.B. (2010). Molecular mechanisms of Escherichia coli pathogenicity. Nat. Rev. Microbiol. 8, 26–38 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

