Insight into human alveolar macrophage and M. tuberculosis interactions via metabolic reconstructions
- PMID: 20959820
- PMCID: PMC2990636
- DOI: 10.1038/msb.2010.68
Insight into human alveolar macrophage and M. tuberculosis interactions via metabolic reconstructions
Abstract
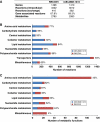
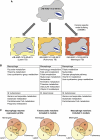
Metabolic coupling of Mycobacterium tuberculosis to its host is foundational to its pathogenesis. Computational genome-scale metabolic models have shown utility in integrating -omic as well as physiologic data for systemic, mechanistic analysis of metabolism. To date, integrative analysis of host-pathogen interactions using in silico mass-balanced, genome-scale models has not been performed. We, therefore, constructed a cell-specific alveolar macrophage model, iAB-AMØ-1410, from the global human metabolic reconstruction, Recon 1. The model successfully predicted experimentally verified ATP and nitric oxide production rates in macrophages. This model was then integrated with an M. tuberculosis H37Rv model, iNJ661, to build an integrated host-pathogen genome-scale reconstruction, iAB-AMØ-1410-Mt-661. The integrated host-pathogen network enables simulation of the metabolic changes during infection. The resulting reaction activity and gene essentiality targets of the integrated model represent an altered infectious state. High-throughput data from infected macrophages were mapped onto the host-pathogen network and were able to describe three distinct pathological states. Integrated host-pathogen reconstructions thus form a foundation upon which understanding the biology and pathophysiology of infections can be developed.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Antohe F, Radulescu L, Puchianu E, Kennedy MD, Low PS, Simionescu M (2005) Increased uptake of folate conjugates by activated macrophages in experimental hyperlipemia. Cell Tissue Res 320: 277–285 - PubMed
-
- Bacon J, James BW, Wernisch L, Williams A, Morley KA, Hatch GJ, Mangan JA, Hinds J, Stoker NG, Butcher PD, Marsh PD (2004) The influence of reduced oxygen availability on pathogenicity and gene expression in Mycobacterium tuberculosis. Tuberculosis (Edinb) 84: 205–217 - PubMed
-
- Becker SA, Feist AM, Mo ML, Hannum G, Palsson BO, Herrgard MJ (2007) Quantitative prediction of cellular metabolism with constraint-based models: the COBRA Toolbox. Nat Protocols 2: 727–738 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

