Nigrostriatal denervation changes the effect of cannabinoids on subthalamic neuronal activity in rats
- PMID: 20959968
- PMCID: PMC3045509
- DOI: 10.1007/s00213-010-2043-0
Nigrostriatal denervation changes the effect of cannabinoids on subthalamic neuronal activity in rats
Abstract
Rationale: It is known that dopaminergic cell loss leads to increased endogenous cannabinoid levels and CB1 receptor density.
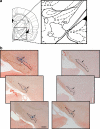
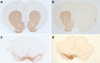
Objective: The aim of this study was to evaluate the influence of dopaminergic cell loss, induced by injection of 6-hydroxydopamine, on the effects exerted by cannabinoid agonists on neuron activity in the subthalamic nucleus (STN) of anesthetized rats.
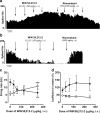
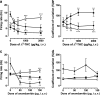
Results: We have previously shown that Δ(9)-tetrahydrocannabinol (Δ(9)-THC) and anandamide induce both stimulation and inhibition of STN neuron activity and that endocannabinoids mediate tonic control of STN activity. Here, we show that in intact rats, the cannabinoid agonist WIN 55,212-2 stimulated all recorded STN neurons. Conversely, after dopaminergic depletion, WIN 55,212-2, Δ(9)-THC, or anandamide inhibited the STN firing rate without altering its discharge pattern, and stimulatory effects were not observed. Moreover, anandamide exerted a more intense inhibitory effect in lesioned rats in comparison to control rats.
Conclusions: Cannabinoids induce different effects on the STN depending on the integrity of the nigrostriatal pathway. These findings advance our understanding of the role of cannabinoids in diseases involving dopamine deficits.
Figures

Similar articles
-
Regulation of subthalamic neuron activity by endocannabinoids.Synapse. 2010 Sep;64(9):682-98. doi: 10.1002/syn.20778. Synapse. 2010. PMID: 20336631
-
Evaluation of the neuroprotective effect of cannabinoids in a rat model of Parkinson's disease: importance of antioxidant and cannabinoid receptor-independent properties.Brain Res. 2007 Feb 23;1134(1):162-70. doi: 10.1016/j.brainres.2006.11.063. Epub 2006 Dec 28. Brain Res. 2007. PMID: 17196181
-
Type 1 cannabinoid receptor ligands display functional selectivity in a cell culture model of striatal medium spiny projection neurons.J Biol Chem. 2014 Sep 5;289(36):24845-62. doi: 10.1074/jbc.M114.557025. Epub 2014 Jul 18. J Biol Chem. 2014. PMID: 25037227 Free PMC article.
-
Cannabinoid-induced changes in respiration of brain mitochondria.Toxicol Lett. 2014 Nov 18;231(1):62-71. doi: 10.1016/j.toxlet.2014.09.002. Epub 2014 Sep 6. Toxicol Lett. 2014. PMID: 25195527
-
Cannabinoid pharmacology: implications for additional cannabinoid receptor subtypes.Chem Phys Lipids. 2002 Dec 31;121(1-2):57-63. doi: 10.1016/s0009-3084(02)00146-9. Chem Phys Lipids. 2002. PMID: 12505690 Review.
Cited by
-
Increased antiparkinson efficacy of the combined administration of VEGF- and GDNF-loaded nanospheres in a partial lesion model of Parkinson's disease.Int J Nanomedicine. 2014 May 27;9:2677-87. doi: 10.2147/IJN.S61940. eCollection 2014. Int J Nanomedicine. 2014. PMID: 24920904 Free PMC article.
-
Morphological Changes in a Severe Model of Parkinson's Disease and Its Suitability to Test the Therapeutic Effects of Microencapsulated Neurotrophic Factors.Mol Neurobiol. 2017 Dec;54(10):7722-7735. doi: 10.1007/s12035-016-0244-1. Epub 2016 Nov 14. Mol Neurobiol. 2017. PMID: 27844282
-
Topographical Distribution of Morphological Changes in a Partial Model of Parkinson's Disease--Effects of Nanoencapsulated Neurotrophic Factors Administration.Mol Neurobiol. 2015 Oct;52(2):846-58. doi: 10.1007/s12035-015-9234-y. Epub 2015 Jun 4. Mol Neurobiol. 2015. PMID: 26041662
-
The role of the subthalamic nucleus in L-DOPA induced dyskinesia in 6-hydroxydopamine lesioned rats.PLoS One. 2012;7(8):e42652. doi: 10.1371/journal.pone.0042652. Epub 2012 Aug 6. PLoS One. 2012. PMID: 22880070 Free PMC article.
-
Nanodelivery of Cerebrolysin and Rearing in Enriched Environment Induce Neuroprotective Effects in a Preclinical Rat Model of Parkinson's Disease.Mol Neurobiol. 2018 Jan;55(1):286-299. doi: 10.1007/s12035-017-0741-x. Mol Neurobiol. 2018. PMID: 28840482
References
-
- Benazzouz A, Piallat B, Ni ZG, Koudsie A, Pollak P, Benabid AL. Implication of the subthalamic nucleus in the pathophysiology and pathogenesis of Parkinson's disease. Cell Transplant. 2000;9:215–221. - PubMed
-
- Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus II Neuronal activity in the MPTP model of Parkinsonism. J Neurophysiol. 1994;72:507–520. - PubMed
-
- Bilbao G, Ruiz-Ortega JA, Miguens N, Ulibarri I, Linazasoro G, Gomez-Urquijo S, Garibi J, Ugedo L. Electrophysiological characterization of substantia nigra dopaminergic neurons in partially lesioned rats: effects of subthalamotomy and levodopa treatment. Brain Res. 2006;1084:175–184. doi: 10.1016/j.brainres.2006.02.052. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

