Structure of the gap junction channel and its implications for its biological functions
- PMID: 20960023
- PMCID: PMC11114897
- DOI: 10.1007/s00018-010-0551-z
Structure of the gap junction channel and its implications for its biological functions
Abstract
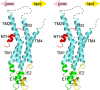
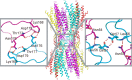
Gap junctions consist of arrays of intercellular channels composed of integral membrane proteins called connexin in vertebrates. Gap junction channels regulate the passage of ions and biological molecules between adjacent cells and, therefore, are critically important in many biological activities, including development, differentiation, neural activity, and immune response. Mutations in connexin genes are associated with several human diseases, such as neurodegenerative disease, skin disease, deafness, and developmental abnormalities. The activity of gap junction channels is regulated by the membrane voltage, intracellular microenvironment, interaction with other proteins, and phosphorylation. Each connexin channel has its own property for conductance and molecular permeability. A number of studies have tried to reveal the molecular architecture of the channel pore that should confer the connexin-specific permeability/selectivity properties and molecular basis for the gating and regulation. In this review, we give an overview of structural studies and describe the structural and functional relationship of gap junction channels.
Figures

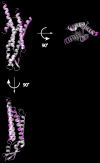


Similar articles
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
-
Structure of the connexin 26 gap junction channel at 3.5 A resolution.Nature. 2009 Apr 2;458(7238):597-602. doi: 10.1038/nature07869. Nature. 2009. PMID: 19340074
-
Analysis of trafficking, stability and function of human connexin 26 gap junction channels with deafness-causing mutations in the fourth transmembrane helix.PLoS One. 2013 Aug 15;8(8):e70916. doi: 10.1371/journal.pone.0070916. eCollection 2013. PLoS One. 2013. PMID: 23967136 Free PMC article.
-
Gating of Connexin Channels by transjunctional-voltage: Conformations and models of open and closed states.Biochim Biophys Acta Biomembr. 2018 Jan;1860(1):22-39. doi: 10.1016/j.bbamem.2017.04.028. Epub 2017 May 2. Biochim Biophys Acta Biomembr. 2018. PMID: 28476631 Free PMC article. Review.
-
Connexin channel permeability to cytoplasmic molecules.Prog Biophys Mol Biol. 2007 May-Jun;94(1-2):120-43. doi: 10.1016/j.pbiomolbio.2007.03.011. Epub 2007 Mar 19. Prog Biophys Mol Biol. 2007. PMID: 17470375 Free PMC article. Review.
Cited by
-
Cataracts and microphthalmia caused by a Gja8 mutation in extracellular loop 2.PLoS One. 2012;7(12):e52894. doi: 10.1371/journal.pone.0052894. Epub 2012 Dec 26. PLoS One. 2012. PMID: 23300808 Free PMC article.
-
Connexins and diabetes.Cardiol Res Pract. 2012;2012:496904. doi: 10.1155/2012/496904. Epub 2012 Mar 1. Cardiol Res Pract. 2012. PMID: 22536530 Free PMC article.
-
Age-related changes in gap junctional intercellular communication in osteoblastic cells.J Orthop Res. 2012 Dec;30(12):1979-84. doi: 10.1002/jor.22172. Epub 2012 Jun 13. J Orthop Res. 2012. PMID: 22696456 Free PMC article.
-
Multifaceted roles of tunneling nanotubes in intercellular communication.Front Physiol. 2012 Apr 10;3:72. doi: 10.3389/fphys.2012.00072. eCollection 2012. Front Physiol. 2012. PMID: 22514537 Free PMC article.
-
The Hydrophobic Residues in Amino Terminal Domains of Cx46 and Cx50 Are Important for Their Gap Junction Channel Ion Permeation and Gating.Int J Mol Sci. 2022 Oct 1;23(19):11605. doi: 10.3390/ijms231911605. Int J Mol Sci. 2022. PMID: 36232905 Free PMC article.
References
-
- Alberts B (2008) Molecular biology of the cell (various pagings). Garland Science, New York, pp 1 v
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

