Preparative and mechanistic studies toward the rational development of catalytic, enantioselective selenoetherification reactions
- PMID: 20961070
- PMCID: PMC2981442
- DOI: 10.1021/ja106837b
Preparative and mechanistic studies toward the rational development of catalytic, enantioselective selenoetherification reactions
Abstract
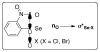
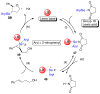
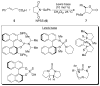
A systematic investigation into the Lewis base catalyzed, asymmetric, intramolecular selenoetherification of olefins is described. A critical challenge for the development of this process was the identification and suppression of racemization pathways available to arylseleniranium ion intermediates. This report details a thorough study of the influences of the steric and electronic modulation of the arylselenenyl group on the configurational stability of enantioenriched seleniranium ions. These studies show that the 2-nitrophenyl group attached to the selenium atom significantly attenuates the racemization of seleniranium ions. A variety of achiral Lewis bases catalyze the intramolecular selenoetherification of alkenes using N-(2-nitrophenylselenenyl)succinimide as the electrophile along with a Brønsted acid. Preliminary mechanistic studies suggest the intermediacy of ionic Lewis base-selenium(II) adducts. Most importantly, a broad survey of chiral Lewis bases revealed that 1,1'-binaphthalene-2,2'-diamine (BINAM)-derived thiophosphoramides catalyze the cyclization of unsaturated alcohols in the presence of N-(2-nitrophenylselenenyl)succinimide and methanesulfonic acid. A variety of cyclic seleno ethers were produced in good chemical yields and in moderate to good enantioselectivities, which constitutes the first catalytic, enantioselective selenofunctionalization of unactivated olefins.
Figures




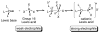











Similar articles
-
Development and mechanism of an enantioselective bromocycloetherification reaction via Lewis base/chiral Brønsted acid cooperative catalysis.Chirality. 2014 Jul;26(7):344-55. doi: 10.1002/chir.22259. Epub 2013 Nov 7. Chirality. 2014. PMID: 24395341 Free PMC article.
-
Enantioselective copper-catalyzed carboetherification of unactivated alkenes.Angew Chem Int Ed Engl. 2014 Jun 16;53(25):6383-7. doi: 10.1002/anie.201402462. Epub 2014 May 5. Angew Chem Int Ed Engl. 2014. PMID: 24798697 Free PMC article.
-
Achiral Cp*Rh(III)/Chiral Lewis Base Cooperative Catalysis for Enantioselective Cyclization via C-H Activation.J Am Chem Soc. 2022 Apr 27;144(16):7058-7065. doi: 10.1021/jacs.2c01223. Epub 2022 Apr 11. J Am Chem Soc. 2022. PMID: 35404054
-
Catalytic, Enantioselective Sulfenofunctionalization of Alkenes: Development and Recent Advances.Angew Chem Int Ed Engl. 2020 Nov 2;59(45):19796-19819. doi: 10.1002/anie.202005920. Epub 2020 Aug 18. Angew Chem Int Ed Engl. 2020. PMID: 32452077 Free PMC article. Review.
-
New developments in enantioselective Brønsted acid catalysis: chiral ion pair catalysis and beyond.Ernst Schering Found Symp Proc. 2007;(2):207-53. Ernst Schering Found Symp Proc. 2007. PMID: 18642527 Review.
Cited by
-
Enantioselective Inter- and Intramolecular Sulfenofunctionalization of Unactivated Cyclic and (Z)-Alkenes.ACS Catal. 2022 Jun 17;12(12):7377-7385. doi: 10.1021/acscatal.2c01232. Epub 2022 Jun 7. ACS Catal. 2022. PMID: 36686398 Free PMC article.
-
Remarkable Alkene-to-Alkene and Alkene-to-Alkyne Transfer Reactions of Selenium Dibromide and PhSeBr. Stereoselective Addition of Selenium Dihalides to Cycloalkenes.Molecules. 2020 Jan 3;25(1):194. doi: 10.3390/molecules25010194. Molecules. 2020. PMID: 31947731 Free PMC article.
-
Halides as versatile anions in asymmetric anion-binding organocatalysis.Beilstein J Org Chem. 2021 Sep 1;17:2270-2286. doi: 10.3762/bjoc.17.145. eCollection 2021. Beilstein J Org Chem. 2021. PMID: 34621390 Free PMC article. Review.
-
Organocatalytic asymmetric selenofunctionalization of tryptamine for the synthesis of hexahydropyrrolo[2,3-b]indole derivatives.Beilstein J Org Chem. 2013 Aug 1;9:1559-64. doi: 10.3762/bjoc.9.177. eCollection 2013. Beilstein J Org Chem. 2013. PMID: 23946855 Free PMC article.
-
Lewis base catalyzed, enantioselective, intramolecular sulfenoamination of olefins.J Am Chem Soc. 2014 Jun 25;136(25):8915-8. doi: 10.1021/ja5046296. Epub 2014 Jun 13. J Am Chem Soc. 2014. PMID: 24926794 Free PMC article.
References
-
- Nicolaou KC, Petasis NA. Selenium in Natural Product Synthesis. CIS; Philadelphia: 1984.
- Paulmier C. Selenium Reagents and Intermediates in Organic Synthesis. Pergamon Press; Oxford: 1986.
- Liotta D, editor. Organoselenium Chemistry. Chap 1–2. Wiley; New York: 1987. pp. 1–163.
- Back TG. In: The Chemistry of Organic Selenium and Tellurium Compounds. Patai S, editor. Vol. 2. Wiley; New York: 1987. pp. 94–215.
- Back TG, editor. In Organoselenium Chemistry - A Practical Approach. Oxford University Press; Oxford: 1999.
- Tiecco M. In: Topics in Current Chemistry: Organoselenium Chemistry. Wirth T, editor. Springer; Heidelberg: 2000. pp. 7–54.
- Petragnani N, Stefani HA, Valduga CJ. Tetrahedron. 2001;57:1411–1448.
- Ranganathan S, Muraleedharan KM, Vaish NK, Jayaraman N. Tetrahedron. 2004;60:5273–5308.
-
- Schmid GH, Garratt DG. Tetrahedron. 1978;34:2869–2872.
- Luh TY, So WH, Cheung KS, Tam SW. J Org Chem. 1985;50:3051–3053.
-
- Hassner A, Amarasekara AS. Tetrahedron Lett. 1987;28:5185–5188.
-
- Denis JN, Vicens J, Krief A. Tetrahedron Lett. 1979;29:2697–2700.
- Scarborough RM, Smith AB, Barnette WE, Nicolaou KC. J Org Chem. 1979;44:1742–1744.
-
- Kozikowski AP, Sorgi KL, Schmiesing RJ. J Chem Soc, Chem Commun. 1980:477–479.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

