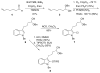
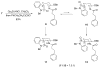
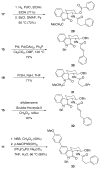
Stereoselective synthesis of spirooxindole amides through nitrile hydrozirconation
- PMID: 20961073
- PMCID: PMC2980567
- DOI: 10.1021/ol102246d
Stereoselective synthesis of spirooxindole amides through nitrile hydrozirconation
Abstract
Spirooxindole amides can be prepared by the intramolecular addition of functionalized indoles into acylimines that are accessed from nitriles by hydrozirconation and acylation. The stereochemical outcome at the quaternary center was controlled by the steric bulk of the substituent at the 2-position of the indole unit. The products are well-suited for diversification to prepare libraries.
Figures
Similar articles
-
Construction of a spirooxindole amide library through nitrile hydrozirconation-acylation-cyclization cascade.ACS Comb Sci. 2013 Jul 8;15(7):344-9. doi: 10.1021/co4000387. Epub 2013 Jun 26. ACS Comb Sci. 2013. PMID: 23731121 Free PMC article.
-
Titanium-catalyzed stereoselective synthesis of spirooxindole oxazolines.Org Lett. 2011 Feb 4;13(3):418-21. doi: 10.1021/ol1027305. Epub 2010 Dec 27. Org Lett. 2011. PMID: 21186788
-
Highly stereoselective Brønsted acid catalyzed synthesis of spirooxindole pyrans.Org Lett. 2011 Jun 17;13(12):3086-9. doi: 10.1021/ol200987c. Epub 2011 May 18. Org Lett. 2011. PMID: 21591768 Free PMC article.
-
FeCl3-catalyzed stereoselective construction of spirooxindole tetrahydroquinolines via tandem 1,5-hydride transfer/ring closure.Org Lett. 2012 Aug 17;14(16):4054-7. doi: 10.1021/ol301559k. Epub 2012 Aug 3. Org Lett. 2012. PMID: 22860987
-
Non-Covalent Organocatalyzed Domino Reactions Involving Oxindoles: Recent Advances.Molecules. 2017 Sep 29;22(10):1636. doi: 10.3390/molecules22101636. Molecules. 2017. PMID: 28961217 Free PMC article. Review.
Cited by
-
Mild Divergent Semireductive Transformations of Secondary and Tertiary Amides via Zirconocene Hydride Catalysis.J Am Chem Soc. 2023 Mar 8;145(9):4921-4927. doi: 10.1021/jacs.2c11786. Epub 2023 Feb 21. J Am Chem Soc. 2023. PMID: 36809854 Free PMC article.
-
Construction of a spirooxindole amide library through nitrile hydrozirconation-acylation-cyclization cascade.ACS Comb Sci. 2013 Jul 8;15(7):344-9. doi: 10.1021/co4000387. Epub 2013 Jun 26. ACS Comb Sci. 2013. PMID: 23731121 Free PMC article.
-
Homoallylic amines by reductive inter- and intramolecular coupling of allenes and nitriles.Beilstein J Org Chem. 2011;7:824-30. doi: 10.3762/bjoc.7.94. Epub 2011 Jun 17. Beilstein J Org Chem. 2011. PMID: 21804878 Free PMC article.
-
Versatile approach to α-alkoxy carbamate synthesis and stimulus-responsive alcohol release.Org Biomol Chem. 2012 Oct 21;10(39):7980-5. doi: 10.1039/c2ob26571k. Org Biomol Chem. 2012. PMID: 22936329 Free PMC article.
-
Stereocontrolled cyanohydrin ether synthesis through chiral Brønsted acid-mediated vinyl ether hydrocyanation.J Org Chem. 2013 Sep 20;78(18):9366-76. doi: 10.1021/jo4016002. Epub 2013 Sep 4. J Org Chem. 2013. PMID: 23968162 Free PMC article.
References
-
- Zhou F, Liu YL, Zhou J. Adv Synth Catal. 2010;352:1381.
- Galliford CV, Scheidt KA. Angew Chem Int Ed. 2007;46:8748. - PubMed
- Marti C, Carreira EM. Eur J Org Chem. 2003:2209.
-
-
For selected recent examples, see: Zhang Y, Panek JS. Org Lett. 2009;11:3366.Shintani R, Hiyashi S-y, Murakami M, Takeda M, Hiyashi T. Org Lett. 2009;11:3754.Bencivenni G, Wu LY, Mazzanti A, Giannichi B, Pesciaioli F, Song MP, Bartoli G, Melchiorre P. Angew Chem Int Ed. 2009;48:7200.Chen XH, Wei Q, Luo SW, Xiao H, Gong LZ. J Am Chem Soc. 2009;131:13819.Liang B, Kalidindi S, Porco JA, Jr, Stephenson CRJ. Org Lett. 2010;12:572.Jiang K, Jia ZJ, Chen S, Wu L, Chen YC. Chem Eur J. 2010;16:2852.Viswambharan B, Selvakumar K, Madhavan S, Shanmugam P. Org Lett. 2010;12:2108.White JD, Li Y, Ihle DC. J Org Chem. 2010;75:3569.Jaegli S, Erb W, Retailleau P, Vors JP, Neuville L, Zhu J. Chem Eur J. 2010;16:5863.Chen WB, Wu ZJ, Pei QL, Cun LF, Zhang XM, Yuan WC. Org Lett. 2010;12:3132.
-
-
- Ding K, Lu Y, Nikolovska-Coleska Z, Qiu S, Ding Y, Gao W, Stuckey J, Krajewski K, Roller PP, Tomita Y, Parrish DA, Deschamps JR, Wang S. J Am Chem Soc. 2005;127:10130. - PubMed
- Yu S, Qin D, Shangary S, Chen J, Wang G, Ding K, McEachern D, Qiu S, Nikolovska-Coleska Z, Miller R, Kang S, Yang D, Wang S. J Med Chem. 2009;52:7970. - PMC - PubMed
-
- Rottmann M, McNamara C, Yeung BKS, Lee MCS, Zou B, Russell B, Seitz P, Plouffe DM, Dharia NV, Tan J, Cohen SB, Spencer KR, González-Páez G, Lakshminarayana SB, Goh A, Suwanarusk R, Jegla T, Schmitt EK, Beck HP, Brun R, Nosten F, Renia L, Dartois V, Keller TH, Fidock DA, Winzeler EA, Diagana TT. Science. 2010;329:1175. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources