Role of intestinal lymphatics in interstitial volume regulation and transmucosal water transport
- PMID: 20961304
- PMCID: PMC2966032
- DOI: 10.1111/j.1749-6632.2010.05709.x
Role of intestinal lymphatics in interstitial volume regulation and transmucosal water transport
Abstract
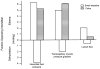
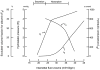
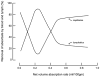
Two of the principal functions of intestinal lymphatics are to assist in the maintenance of interstitial volume within relatively normal limits during alterations in capillary filtration (e.g., acute portal hypertension) and the removal of absorbed water and chylomicrons. The contribution of lymphatics to the prevention of interstitial overhydration or dehydration during alterations in transcapillary filtration is similar in the small intestine and colon. While the lymphatics of the small intestine contribute substantially to the removal of absorbed water (particularly at low and moderate absorption rates), the contribution of colonic lymphatics to the removal of the fluid absorbate is negligible. This difference is attributed to the relative caliber and location of lymphatics in the mucosal layer of the small and large intestines. In the small intestine, large lacteals lie in close proximity to transporting epithelium, while colonic lymph vessels are rather sparse and confined to the basal portion of the mucosa. In the small intestine, the lymphatics assume a more important role in removing absorbed water during lipid absorption than during glucose absorption.
Keywords: colon; lymphatics; secretion; small intestine; transcapillary fluid exchange; water absorption.
© 2010 New York Academy of Sciences.
Figures






References
-
- GRANGER DN, BARROWMAN JA. Microcirculation of the alimentary tract I. Physiology of transcapillary fluid and solute exchange. Gastroenterology. 1983;84:846–868. - PubMed
-
- CASLEY-SMITH J, GANNON BJ. Intestinal microcirculation: spatial organization and fine structure. In: Shepherd A, Granger D, editors. Physiology of the Intestinal Circulation. Raven Press; New York: 1984. pp. 9–31.
-
- GRANGER DN, KVIETYS PR, PERRY MA, BARROWMAN JA. The microcirculation and intestinal transport. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. 2. New York: Raven Press; 1987. pp. 1671–1697.
-
- ARAKI K, FURUYA Y, KOBAYASHI M, MATSUURA K, OGATA T, ISOZAKI H. Comparison of mucosal microvasculature between the proximal and distal human colon 1. J Electron Microsc. 1996;45:202–206. - PubMed
-
- KVIETYS PR, GRANGER DN. Physiology, pharmacology and pathology of the colonic circulation. In: Shepherd AP, Granger DN, editors. Physiology of the Intestinal Circulation. New York: Raven Press; 1984. pp. 131–142.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

