Intranasal vaccination with messenger RNA as a new approach in gene therapy: use against tuberculosis
- PMID: 20961459
- PMCID: PMC2972232
- DOI: 10.1186/1472-6750-10-77
Intranasal vaccination with messenger RNA as a new approach in gene therapy: use against tuberculosis
Abstract
Background: mRNAs are highly versatile, non-toxic molecules that are easy to produce and store, which can allow transient protein expression in all cell types. The safety aspects of mRNA-based treatments in gene therapy make this molecule one of the most promising active components of therapeutic or prophylactic methods. The use of mRNA as strategy for the stimulation of the immune system has been used mainly in current strategies for the cancer treatment but until now no one tested this molecule as vaccine for infectious disease.
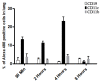
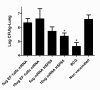
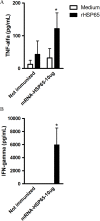
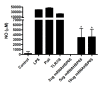
Results: We produce messenger RNA of Hsp65 protein from Mycobacterium leprae and show that vaccination of mice with a single dose of 10 μg of naked mRNA-Hsp65 through intranasal route was able to induce protection against subsequent challenge with virulent strain of Mycobacterium tuberculosis. Moreover it was shown that this immunization was associated with specific production of IL-10 and TNF-alpha in spleen. In order to determine if antigen presenting cells (APCs) present in the lung are capable of capture the mRNA, labeled mRNA-Hsp65 was administered by intranasal route and lung APCs were analyzed by flow cytometry. These experiments showed that after 30 minutes until 8 hours the populations of CD11c+, CD11b+ and CD19+ cells were able to capture the mRNA. We also demonstrated in vitro that mRNA-Hsp65 leads nitric oxide (NO) production through Toll-like receptor 7 (TLR7).
Conclusions: Taken together, our results showed a novel and efficient strategy to control experimental tuberculosis, besides opening novel perspectives for the use of mRNA in vaccines against infectious diseases and clarifying the mechanisms involved in the disease protection we noticed as well.
Figures


References
-
- Weide B, Pascolo S, Scheel B, Derhovanessian E, Pflugfelder A, Eigentler TK, Pawelec G, Hoerr I, Rammensee HG, Garbe C. Direct injection of protamine-protected mRNA: results of a phase 1/2 vaccination trial in metastatic melanoma patients. J Immunother. 2009;32(5):498–507. doi: 10.1097/CJI.0b013e3181a00068. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

