Cholesterol and lipid phases influence the interactions between serotonin receptor agonists and lipid bilayers
- PMID: 20961857
- PMCID: PMC3009866
- DOI: 10.1074/jbc.M110.155176
Cholesterol and lipid phases influence the interactions between serotonin receptor agonists and lipid bilayers
Abstract
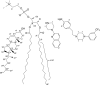
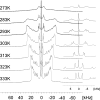
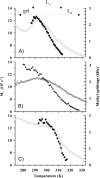
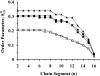
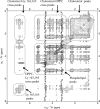
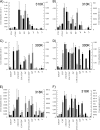
Solid state NMR techniques have been used to investigate the effect that two serotonin receptor 1a agonists (quipazine and LY-165,163) have on the phase behavior of, and interactions within, cholesterol/phosphocholine lipid bilayers. The presence of agonist, and particularly LY-165,163, appears to widen the phase transitions, an effect that is much more pronounced in the presence of cholesterol. It was found that both agonists locate close to the cholesterol, and their interactions with the lipids are modulated by the lipid phases. As the membrane condenses into mixed liquid-ordered/disordered phases, quipazine is pushed up toward the surface of the bilayer, whereas LY-165,163 moves deeper into the lipid chain region. In light of our results, we discuss the role of lipid/drug interactions on drug efficacy.
Figures


Similar articles
-
Location and orientation of serotonin receptor 1a agonists in model and complex lipid membranes.J Biol Chem. 2008 Mar 21;283(12):7813-22. doi: 10.1074/jbc.M707480200. Epub 2008 Jan 9. J Biol Chem. 2008. PMID: 18187421
-
Lateral diffusion coefficients of separate lipid species in a ternary raft-forming bilayer: a Pfg-NMR multinuclear study.Biophys J. 2005 Jul;89(1):315-20. doi: 10.1529/biophysj.105.061762. Epub 2005 Apr 29. Biophys J. 2005. PMID: 15863478 Free PMC article.
-
From lanosterol to cholesterol: structural evolution and differential effects on lipid bilayers.Biophys J. 2002 Mar;82(3):1429-44. doi: 10.1016/S0006-3495(02)75497-0. Biophys J. 2002. PMID: 11867458 Free PMC article.
-
Cholesterol Effects on the Physical Properties of Lipid Membranes Viewed by Solid-state NMR Spectroscopy.Adv Exp Med Biol. 2019;1115:99-133. doi: 10.1007/978-3-030-04278-3_5. Adv Exp Med Biol. 2019. PMID: 30649757 Review.
-
Lipid-protein interplay and lateral organization in biomembranes.Chem Phys Lipids. 2015 Jul;189:48-55. doi: 10.1016/j.chemphyslip.2015.05.008. Epub 2015 May 30. Chem Phys Lipids. 2015. PMID: 26036778 Review.
Cited by
-
Cellular membrane phospholipids act as a depository for quaternary amine containing drugs thus competing with the acetylcholine/nicotinic receptor.J Proteome Res. 2012 Jun 1;11(6):3382-9. doi: 10.1021/pr300184g. Epub 2012 Apr 30. J Proteome Res. 2012. PMID: 22506649 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

