Ca²+ spark-dependent and -independent sarcoplasmic reticulum Ca²+ leak in normal and failing rabbit ventricular myocytes
- PMID: 20962003
- PMCID: PMC3010143
- DOI: 10.1113/jphysiol.2010.197913
Ca²+ spark-dependent and -independent sarcoplasmic reticulum Ca²+ leak in normal and failing rabbit ventricular myocytes
Abstract
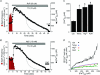
Sarcoplasmic reticulum (SR) Ca²(+) leak is an important component of cardiac Ca²(+) signalling. Together with the SR Ca²(+)-ATPase (SERCA)-mediated Ca²(+) uptake, diastolic Ca²(+) leak determines SR Ca²(+) load and, therefore, the amplitude of Ca²(+) transients that initiate contraction. Spontaneous Ca²(+) sparks are thought to play a major role in SR Ca²(+) leak. In this study, we determined the quantitative contribution of sparks to SR Ca²(+) leak and tested the hypothesis that non-spark mediated Ca²(+) release also contributes to SR Ca²(+) leak. We simultaneously measured spark properties and intra-SR free Ca²(+) ([Ca²(+)](SR)) after complete inhibition of SERCA with thapsigargin in permeabilized rabbit ventricular myocytes. When [Ca²(+)](SR) declined to 279 ± 10 μm, spark activity ceased completely; however SR Ca²(+) leak continued, albeit at a slower rate. Analysis of sparks and [Ca²(+)](SR) revealed, that SR Ca²(+) leak increased as a function of [Ca²(+)](SR), with a particularly steep increase at higher [Ca²(+)](SR) ( >600 μm) where sparks become a major pathway of SR Ca²(+) leak. At low [Ca²(+)](SR) (< 300 μm), however, Ca²(+) leak occurred mostly as non-spark-mediated leak. Sensitization of ryanodine receptors (RyRs) with low doses of caffeine increased spark frequency and SR Ca²(+) leak. Complete inhibition of RyR abolished sparks and significantly decreased SR Ca²(+) leak, but did not prevent it entirely, suggesting the existence of RyR-independent Ca²(+) leak. Finally, we found that RyR-mediated Ca²(+) leak was enhanced in myocytes from failing rabbit hearts. These results show that RyRs are the main, but not sole contributor to SR Ca²(+) leak. RyR-mediated leak occurs in part as Ca²(+) sparks, but there is clearly RyR-mediated but Ca²(+) sparks independent leak.
Figures
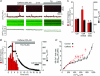
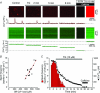
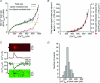
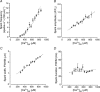

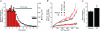
Comment in
-
A small leak may sink a great ship but what does it do to the heart?J Physiol. 2010 Dec 15;588(Pt 24):4849. doi: 10.1113/jphysiol.2010.200840. J Physiol. 2010. PMID: 21173084 Free PMC article. No abstract available.
References
-
- Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97:1314–1322. - PubMed
-
- Bassani JW, Yuan W, Bers DM. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am J Physiol Cell Physiol. 1995;268:C1313–C1319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

