Effects of acidification and increased extracellular potassium on dynamic muscle contractions in isolated rat muscles
- PMID: 20962010
- PMCID: PMC3036197
- DOI: 10.1113/jphysiol.2010.195727
Effects of acidification and increased extracellular potassium on dynamic muscle contractions in isolated rat muscles
Abstract
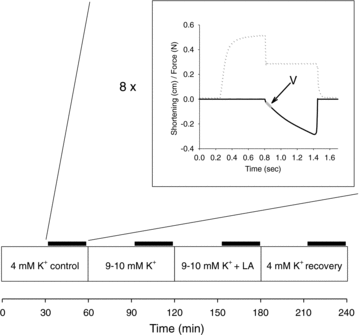
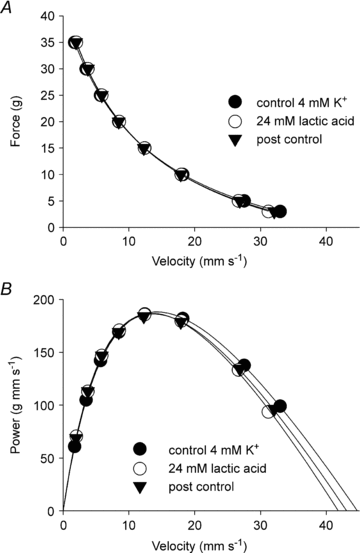
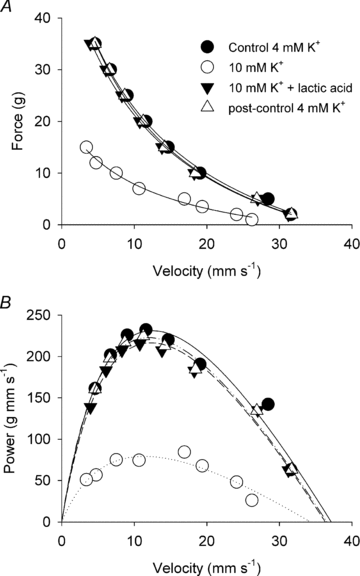
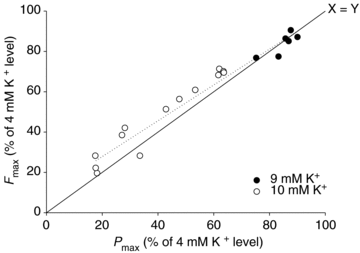
Since accumulation of both H(+) and extracellular K(+) have been implicated in the reduction in dynamic contractile function during intense exercise, we investigated the effects of acidification and high K(+) on muscle power and the force-velocity relation in non-fatigued rat soleus muscles. Contractions were elicited by supramaximal electrical stimulation at 60 Hz. Force-velocity (FV) curves were obtained by fitting data on force and shortening velocity at different loads to the Hill equation. Acidification of the muscles by incubation with up to 24 mm lactic acid produced no significant changes in maximal power (P(max)) at 30 °C. More pronounced acidification, obtained by increasing CO(2) levels in the equilibration gas from 5% to 53%, markedly decreased P(max) and maximal isometric force (F(max)), increased the curvature of the FV relation, but left maximal shortening velocity (V(max)) unchanged. Increase of extracellular K(+) from 4 to 10 mm caused a depression of 58% in P(max) and 52% in F(max), but had no significant effect on V(max) or curvature of the FV curve. When muscles at 10 mM K(+) were acidified by 20 mm lactic acid, P(max) and F(max) recovered completely to the initial control level at 4 mm K(+). CO(2) acidification also induced significant recovery of dynamic contractions, but not entirely to control levels. These results demonstrate that in non-fatigued muscles severe acidification can be detrimental to dynamic contractile function, but in muscles depolarised by exposure to high extracellular [K(+)], approaching the [K(+)] level seen during intense fatiguing exercise, acidification can have positive protective effects on dynamic muscle function.
Figures

Similar articles
-
Fatiguing stimulation increases curvature of the force-velocity relationship in isolated fast-twitch and slow-twitch rat muscles.J Exp Biol. 2019 Aug 9;222(Pt 15):jeb204545. doi: 10.1242/jeb.204545. J Exp Biol. 2019. PMID: 31292165
-
Protective effects of lactic acid on force production in rat skeletal muscle.J Physiol. 2001 Oct 1;536(Pt 1):161-6. doi: 10.1111/j.1469-7793.2001.t01-1-00161.x. J Physiol. 2001. PMID: 11579166 Free PMC article.
-
Additive protective effects of the addition of lactic acid and adrenaline on excitability and force in isolated rat skeletal muscle depressed by elevated extracellular K+.J Physiol. 2007 Jun 1;581(Pt 2):829-39. doi: 10.1113/jphysiol.2007.129049. Epub 2007 Mar 8. J Physiol. 2007. PMID: 17347268 Free PMC article.
-
Lactic acid and exercise performance : culprit or friend?Sports Med. 2006;36(4):279-91. doi: 10.2165/00007256-200636040-00001. Sports Med. 2006. PMID: 16573355 Review.
-
Ion gradients and contractility in skeletal muscle: the role of active Na+, K+ transport.Acta Physiol Scand. 1996 Mar;156(3):247-56. doi: 10.1046/j.1365-201X.1996.204000.x. Acta Physiol Scand. 1996. PMID: 8729684 Review.
Cited by
-
Mechanistic Insights into the Efficacy of Sodium Bicarbonate Supplementation to Improve Athletic Performance.Sports Med Open. 2016 Dec;2(1):41. doi: 10.1186/s40798-016-0065-9. Epub 2016 Oct 11. Sports Med Open. 2016. PMID: 27747796 Free PMC article. Review.
-
Associations between skeletal muscle phenotype, positional role, and on-ice performance in elite male ice hockey players.Physiol Rep. 2024 Nov;12(21):e70081. doi: 10.14814/phy2.70081. Physiol Rep. 2024. PMID: 39523499 Free PMC article.
-
Lactic acidosis: implications for human exercise performance.Eur J Appl Physiol. 2025 Jul;125(7):1761-1795. doi: 10.1007/s00421-025-05750-0. Epub 2025 Mar 15. Eur J Appl Physiol. 2025. PMID: 40088272 Free PMC article. Review.
-
Effects of high-frequency stimulation and doublets on dynamic contractions in rat soleus muscle exposed to normal and high extracellular [K(+)].Physiol Rep. 2013 Jul;1(2):e00026. doi: 10.1002/phy2.26. Epub 2013 Jul 15. Physiol Rep. 2013. PMID: 24303113 Free PMC article.
-
Enhancing team-sport athlete performance: is altitude training relevant?Sports Med. 2012 Sep 1;42(9):751-67. doi: 10.1007/BF03262293. Sports Med. 2012. PMID: 22845561 Review.
References
-
- Adams GR, Fisher MJ, Meyer RA. Hypercapnic acidosis and increased H2PO4− concentration do not decrease force in cat skeletal muscle. Am J Physiol Cell Physiol. 1991;260:C805–C812. - PubMed
-
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88:287–332. - PubMed
-
- Bar A, Pette D. Three fast myosin heavy chains in adult rat skeletal muscle. FEBS Lett. 1988;235:153–155. - PubMed
-
- Broch-Lips M, Overgaard K, Praetorius HA, Nielsen OB. Effects of extracellular HCO3− on fatigue, pHi, and K+ efflux in rat skeletal muscles. J Appl Physiol. 2007;103:494–503. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

