Voltage-dependent block by internal spermine of the murine inwardly rectifying K+ channel, Kir2.1, with asymmetrical K+ concentrations
- PMID: 20962011
- PMCID: PMC3010137
- DOI: 10.1113/jphysiol.2010.194480
Voltage-dependent block by internal spermine of the murine inwardly rectifying K+ channel, Kir2.1, with asymmetrical K+ concentrations
Abstract
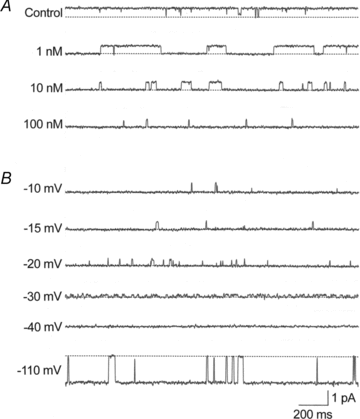
Effects of internal spermine on outward single-channel currents through a strongly inwardly rectifying K(+) channel (Kir2.1) were studied at asymmetrical K(+) concentrations (30 mm external and 150 mm internal K(+)). The current-voltage (I-V) relation for the single channel was almost linear and reversed at -37 ± 3 mV (V(R); n = 19). The channel conductance was 26.3 ± 1.3 pS (n = 24). The open-time and closed-time histograms were fitted with a single exponential function. Internal spermine at a concentration of 1-100 nm reduced the open time of the outward currents in a concentration-dependent manner and produced a blocked state. The steady-state open probability of the outward current decreased with larger depolarizations in both the absence and presence of internal spermine. The steady-state open probability with asymmetrical K(+) and symmetrical (150 mm external and internal K(+)) concentrations plotted against driving force (V - V(R)) coincided with smaller depolarizations in the absence of spermine and larger depolarizations and higher spermine concentrations in the presence of spermine. The blocking rate constants and unblock rates with 30 mm and 150 mm external K(+) were similar at the same driving force. The dissociation constant-membrane potential relation for 30 mm external K(+) was shifted in the negative direction from that for 150 mm external K(+) by 36 mV. These results suggested that the blocking kinetics depends on driving force to produce driving force-dependent inward rectification when the equilibrium potential for K(+) is altered by changing external K(+) and that the energy barriers and wells for blocking ions from passing or lodging are not stable but affected by external K(+) ions.
Figures



Similar articles
-
Voltage-dependent gating and block by internal spermine of the murine inwardly rectifying K+ channel, Kir2.1.J Physiol. 2003 Apr 15;548(Pt 2):361-71. doi: 10.1113/jphysiol.2003.038844. Epub 2003 Mar 14. J Physiol. 2003. PMID: 12640008 Free PMC article.
-
Effects of external and internal K+ ions on magnesium block of inwardly rectifying K+ channels in guinea-pig heart cells.J Physiol. 1991 Apr;435:83-99. doi: 10.1113/jphysiol.1991.sp018499. J Physiol. 1991. PMID: 1770455 Free PMC article.
-
Low-affinity spermine block mediating outward currents through Kir2.1 and Kir2.2 inward rectifier potassium channels.J Physiol. 2007 Sep 15;583(Pt 3):891-908. doi: 10.1113/jphysiol.2007.136028. Epub 2007 Jul 19. J Physiol. 2007. PMID: 17640933 Free PMC article.
-
Effects of internal and external Na+ ions on inwardly rectifying K+ channels in guinea-pig ventricular cells.J Physiol. 1993 Jan;460:311-26. doi: 10.1113/jphysiol.1993.sp019473. J Physiol. 1993. PMID: 8487197 Free PMC article.
-
Two Kir2.1 channel populations with different sensitivities to Mg(2+) and polyamine block: a model for the cardiac strong inward rectifier K(+) channel.J Physiol. 2005 Mar 15;563(Pt 3):725-44. doi: 10.1113/jphysiol.2004.079186. Epub 2004 Dec 23. J Physiol. 2005. PMID: 15618275 Free PMC article.
Cited by
-
Waixenicin A inhibits cell proliferation through magnesium-dependent block of transient receptor potential melastatin 7 (TRPM7) channels.J Biol Chem. 2011 Nov 11;286(45):39328-35. doi: 10.1074/jbc.M111.264341. Epub 2011 Sep 16. J Biol Chem. 2011. PMID: 21926172 Free PMC article.
-
A synergistic blocking effect of Mg²⁺ and spermine on the inward rectifier K⁺ (Kir2.1) channel pore.Sci Rep. 2016 Feb 12;6:21493. doi: 10.1038/srep21493. Sci Rep. 2016. PMID: 26869275 Free PMC article.
-
Linkage analysis reveals allosteric coupling in Kir2.1 channels.J Gen Physiol. 2018 Nov 5;150(11):1541-1553. doi: 10.1085/jgp.201812127. Epub 2018 Oct 16. J Gen Physiol. 2018. PMID: 30327330 Free PMC article.
-
The bundle crossing region is responsible for the inwardly rectifying internal spermine block of the Kir2.1 channel.Pflugers Arch. 2014 Feb;466(2):275-93. doi: 10.1007/s00424-013-1322-0. Epub 2013 Jul 20. Pflugers Arch. 2014. PMID: 23873351
References
-
- Ficker E, Taglialatela M, Wible BA, Henley CM, Brown AM. Spermine and spermidine as gating molecules for inward rectifier K+ channels. Science. 1994;266:1068–1072. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

