The nitric oxide prodrug JS-K is effective against non-small-cell lung cancer cells in vitro and in vivo: involvement of reactive oxygen species
- PMID: 20962031
- PMCID: PMC3033717
- DOI: 10.1124/jpet.110.174904
The nitric oxide prodrug JS-K is effective against non-small-cell lung cancer cells in vitro and in vivo: involvement of reactive oxygen species
Abstract
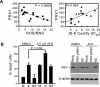
Non-small-cell lung cancer is among the most common and deadly forms of human malignancies. Early detection is unusual, and there are no curative therapies in most cases. Diazeniumdiolate-based nitric oxide (NO)-releasing prodrugs are a growing class of promising NO-based therapeutics. Here, we show that O(2)-(2,4-dinitrophenyl)-1-[(4-ethoxycarbonyl)piperazin-1-yl]diazen-1-ium-1,2-diolate (JS-K) is a potent cytotoxic agent against a subset of human non-small-cell lung cancer cell lines both in vitro and as xenografts in mice. JS-K treatment led to 75% reduction in the growth of H1703 lung adenocarcinoma cells in vivo. Differences in sensitivity to JS-K in different lung cancer cell lines seem to be related to their endogenous levels of reactive oxygen species (ROS)/reactive nitrogen species (RNS). Other related factors, levels of peroxiredoxin 1 (PRX1) and 8-oxo-deoxyguanosine glycosylase (OGG1), also correlated with drug sensitivity. Treatment of the lung adenocarcinoma cells with JS-K resulted in oxidative/nitrosative stress in cells with high basal levels of ROS/RNS, which, combined with the arylating properties of the compound, was reflected in glutathione depletion and alteration in cellular redox potential, mitochondrial membrane permeabilization, and cytochrome c release. Inactivation of manganese superoxide dismutase by nitration was associated with increased superoxide and significant DNA damage. Apoptosis followed these events. Taken together, the data suggest that diazeniumdiolate-based NO-releasing prodrugs may have application as a personalized therapy for lung cancers characterized by high levels of ROS/RNS. PRX1 and OGG1 proteins, which can be easily measured, could function as biomarkers for identifying tumors sensitive to the therapy.
Figures

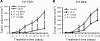
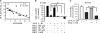
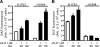


References
-
- Bentz BG, Hammer ND, Milash B, Klein S, Burnett DM, Radosevich JA, Haines GK., 3rd (2007) The kinetics and redox state of nitric oxide determine the biological consequences in lung adenocarcinoma. Tumour Biol 28:301–311 - PubMed
-
- Bonavida B, Baritaki S, Huerta-Yepez S, Vega MI, Chatterjee D, Yeung K. (2008) Novel therapeutic applications of nitric oxide donors in cancer: roles in chemo- and immunosensitization to apoptosis and inhibition of metastases. Nitric Oxide 19:152–157 - PubMed
-
- Bonavida B, Khineche S, Huerta-Yepez S, Garbán H. (2006) Therapeutic potential of nitric oxide in cancer. Drug Resist Updat 9:157–173 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

