Serum-stable RNA aptamers to urokinase-type plasminogen activator blocking receptor binding
- PMID: 20962041
- PMCID: PMC2995398
- DOI: 10.1261/rna.2338210
Serum-stable RNA aptamers to urokinase-type plasminogen activator blocking receptor binding
Abstract
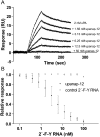
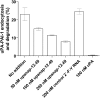
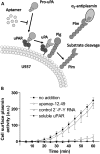
The serine proteinase urokinase-type plasminogen activator (uPA) is widely recognized as a potential target for anticancer therapy. Its association with cell surfaces through the uPA receptor (uPAR) is central to its function and plays an important role in cancer invasion and metastasis. In the current study, we used systematic evolution of ligands by exponential enrichment (SELEX) to select serum-stable 2'-fluoro-pyrimidine-modified RNA aptamers specifically targeting human uPA and blocking the interaction to its receptor at low nanomolar concentrations. In agreement with the inhibitory function of the aptamers, binding was found to be dependent on the presence of the growth factor domain of uPA, which mediates uPAR binding. One of the most potent uPA aptamers, upanap-12, was analyzed in more detail and could be reduced significantly in size without severe loss of its inhibitory activity. Finally, we show that the uPA-scavenging effect of the aptamers can reduce uPAR-dependent endocytosis of the uPA-PAI-1 complex and cell-surface associated plasminogen activation in cell culture experiments. uPA-scavenging 2'-fluoro-pyrimidine-modified RNA aptamers represent a novel promising principle for interfering with the pathological functions of the uPA system.
Figures


Similar articles
-
Targeting tumor cell invasion and dissemination in vivo by an aptamer that inhibits urokinase-type plasminogen activator through a novel multifunctional mechanism.Mol Cancer Res. 2012 Dec;10(12):1532-43. doi: 10.1158/1541-7786.MCR-12-0349. Epub 2012 Oct 4. Mol Cancer Res. 2012. PMID: 23038812 Free PMC article.
-
Inhibition of Human Urokinase-Type Plasminogen Activator (uPA) Enzyme Activity and Receptor Binding by DNA Aptamers as Potential Therapeutics through Binding to the Different Forms of uPA.Int J Mol Sci. 2022 Apr 28;23(9):4890. doi: 10.3390/ijms23094890. Int J Mol Sci. 2022. PMID: 35563278 Free PMC article.
-
Regions involved in binding of urokinase-type-1 inhibitor complex and pro-urokinase to the endocytic alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein. Evidence that the urokinase receptor protects pro-urokinase against binding to the endocytic receptor.J Biol Chem. 1994 Oct 14;269(41):25668-76. J Biol Chem. 1994. PMID: 7929271
-
Interference with the urokinase plasminogen activator system: a promising therapy concept for solid tumours.Expert Opin Biol Ther. 2001 Jul;1(4):683-91. doi: 10.1517/14712598.1.4.683. Expert Opin Biol Ther. 2001. PMID: 11727504 Review.
-
Urokinase plasminogen activator system as a potential target for cancer therapy.Future Oncol. 2009 Nov;5(9):1487-99. doi: 10.2217/fon.09.108. Future Oncol. 2009. PMID: 19903074 Review.
Cited by
-
Targeting tumor cell invasion and dissemination in vivo by an aptamer that inhibits urokinase-type plasminogen activator through a novel multifunctional mechanism.Mol Cancer Res. 2012 Dec;10(12):1532-43. doi: 10.1158/1541-7786.MCR-12-0349. Epub 2012 Oct 4. Mol Cancer Res. 2012. PMID: 23038812 Free PMC article.
-
Inhibition of Human Urokinase-Type Plasminogen Activator (uPA) Enzyme Activity and Receptor Binding by DNA Aptamers as Potential Therapeutics through Binding to the Different Forms of uPA.Int J Mol Sci. 2022 Apr 28;23(9):4890. doi: 10.3390/ijms23094890. Int J Mol Sci. 2022. PMID: 35563278 Free PMC article.
-
A serum-stable RNA aptamer specific for SARS-CoV-2 neutralizes viral entry.Proc Natl Acad Sci U S A. 2021 Dec 14;118(50):e2112942118. doi: 10.1073/pnas.2112942118. Proc Natl Acad Sci U S A. 2021. PMID: 34876524 Free PMC article.
-
Differential RNA aptamer affinity profiling on plasma as a potential diagnostic tool for bladder cancer.NAR Cancer. 2022 Aug 22;4(3):zcac025. doi: 10.1093/narcan/zcac025. eCollection 2022 Sep. NAR Cancer. 2022. PMID: 36004048 Free PMC article.
-
An isothermal system that couples ligand-dependent catalysis to ligand-independent exponential amplification.J Am Chem Soc. 2011 Mar 9;133(9):3191-7. doi: 10.1021/ja111136d. Epub 2011 Feb 15. J Am Chem Soc. 2011. PMID: 21322594 Free PMC article.
References
-
- Alfano D, Iaccarino I, Stoppelli MP 2006. Urokinase signaling through its receptor protects against anoikis by increasing BCL-xL expression levels. J Biol Chem 281: 17758–17767 - PubMed
-
- Andreasen PA, Kjoller L, Christensen L, Duffy MJ 1997. The urokinase-type plasminogen activator system in cancer metastasis: A review. Int J Cancer 72: 1–22 - PubMed
-
- Carriero MV, Del Vecchio S, Capozzoli M, Franco P, Fontana L, Zannetti A, Botti G, D'Aiuto G, Salvatore M, Stoppelli MP 1999. Urokinase receptor interacts with αvβ5 vitronectin receptor, promoting urokinase-dependent cell migration in breast cancer. Cancer Res 59: 5307–5314 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
