Serum-stable RNA aptamers to urokinase-type plasminogen activator blocking receptor binding
- PMID: 20962041
- PMCID: PMC2995398
- DOI: 10.1261/rna.2338210
Serum-stable RNA aptamers to urokinase-type plasminogen activator blocking receptor binding
Abstract
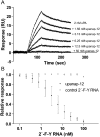
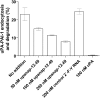
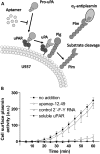
The serine proteinase urokinase-type plasminogen activator (uPA) is widely recognized as a potential target for anticancer therapy. Its association with cell surfaces through the uPA receptor (uPAR) is central to its function and plays an important role in cancer invasion and metastasis. In the current study, we used systematic evolution of ligands by exponential enrichment (SELEX) to select serum-stable 2'-fluoro-pyrimidine-modified RNA aptamers specifically targeting human uPA and blocking the interaction to its receptor at low nanomolar concentrations. In agreement with the inhibitory function of the aptamers, binding was found to be dependent on the presence of the growth factor domain of uPA, which mediates uPAR binding. One of the most potent uPA aptamers, upanap-12, was analyzed in more detail and could be reduced significantly in size without severe loss of its inhibitory activity. Finally, we show that the uPA-scavenging effect of the aptamers can reduce uPAR-dependent endocytosis of the uPA-PAI-1 complex and cell-surface associated plasminogen activation in cell culture experiments. uPA-scavenging 2'-fluoro-pyrimidine-modified RNA aptamers represent a novel promising principle for interfering with the pathological functions of the uPA system.
Figures


References
-
- Alfano D, Iaccarino I, Stoppelli MP 2006. Urokinase signaling through its receptor protects against anoikis by increasing BCL-xL expression levels. J Biol Chem 281: 17758–17767 - PubMed
-
- Andreasen PA, Kjoller L, Christensen L, Duffy MJ 1997. The urokinase-type plasminogen activator system in cancer metastasis: A review. Int J Cancer 72: 1–22 - PubMed
-
- Carriero MV, Del Vecchio S, Capozzoli M, Franco P, Fontana L, Zannetti A, Botti G, D'Aiuto G, Salvatore M, Stoppelli MP 1999. Urokinase receptor interacts with αvβ5 vitronectin receptor, promoting urokinase-dependent cell migration in breast cancer. Cancer Res 59: 5307–5314 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
