Induction of type I interferon secretion through recombinant Newcastle disease virus expressing measles virus hemagglutinin stimulates antibody secretion in the presence of maternal antibodies
- PMID: 20962092
- PMCID: PMC3014200
- DOI: 10.1128/JVI.01624-10
Induction of type I interferon secretion through recombinant Newcastle disease virus expressing measles virus hemagglutinin stimulates antibody secretion in the presence of maternal antibodies
Abstract
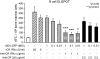
Measles virus (MV) vaccine effectively protects seronegative individuals against infection. However, inhibition of vaccine-induced seroconversion by maternal antibodies after vaccination remains a problem, as it leaves infants susceptible to MV infection. In cotton rats, passive transfer of MV-specific IgG mimics maternal antibodies and inhibits vaccine-induced seroconversion. Here, we report that immunization in the presence of passively transferred IgG inhibits the secretion of neutralizing antibodies but not the generation of MV-specific B cells. This finding suggested that MV-specific B cells require an additional stimulus to mature into antibody-secreting plasma cells. In order to provide such a stimulus, we generated a recombinant Newcastle disease virus (NDV) expressing the MV hemagglutinin (NDV-H). In contrast to MV, NDV-H induced high levels of type I interferon in plasmacytoid dendritic cells and in lung tissue. In cotton rats immunized with NDV-H, neutralizing antibodies were also generated in the presence of passively transferred antibodies. In the latter case, however, the level and kinetics of antibody generation were reduced. In vitro, alpha interferon stimulated the activation of MV-specific B cells from MV-immune spleen cells. NDV infection (which induces alpha interferon) had the same effect, and stimulation could be abrogated by antibodies neutralizing alpha interferon, but not interleukin 6 (IL-6). In vivo, coapplication of UV-inactivated MV with NDV led to increased MV-specific antibody production in the presence and absence of passively transferred antibodies. These data indicate that MV-specific B cells are being generated after immunization in the presence of maternal antibodies and that the provision of alpha interferon as an additional signal leads to antibody secretion.
Figures




Similar articles
-
Synergistic induction of interferon α through TLR-3 and TLR-9 agonists stimulates immune responses against measles virus in neonatal cotton rats.Vaccine. 2014 Jan 3;32(2):265-70. doi: 10.1016/j.vaccine.2013.11.013. Epub 2013 Nov 18. Vaccine. 2014. PMID: 24262312 Free PMC article.
-
Successful vaccine-induced seroconversion by single-dose immunization in the presence of measles virus-specific maternal antibodies.J Virol. 2000 May;74(10):4652-7. doi: 10.1128/jvi.74.10.4652-4657.2000. J Virol. 2000. PMID: 10775601 Free PMC article.
-
Successful mucosal immunization of cotton rats in the presence of measles virus-specific antibodies depends on degree of attenuation of vaccine vector and virus dose.J Gen Virol. 2003 Aug;84(Pt 8):2145-2151. doi: 10.1099/vir.0.19050-0. J Gen Virol. 2003. PMID: 12867646
-
Neutralizing B cell response in measles.Viral Immunol. 2002;15(3):451-71. doi: 10.1089/088282402760312331. Viral Immunol. 2002. PMID: 12479395 Review.
-
Immune responses of poultry to Newcastle disease virus.Dev Comp Immunol. 2013 Nov;41(3):447-53. doi: 10.1016/j.dci.2013.04.012. Epub 2013 Apr 25. Dev Comp Immunol. 2013. PMID: 23623955 Review.
Cited by
-
Success of measles virotherapy in ATL depends on type I interferon secretion and responsiveness.Virus Res. 2014 Aug 30;189:206-13. doi: 10.1016/j.virusres.2014.05.025. Epub 2014 Jun 6. Virus Res. 2014. PMID: 24911240 Free PMC article.
-
Comparing the Primary and Recall Immune Response Induced by a New EV71 Vaccine Using Systems Biology Approaches.PLoS One. 2015 Oct 14;10(10):e0140515. doi: 10.1371/journal.pone.0140515. eCollection 2015. PLoS One. 2015. PMID: 26465882 Free PMC article.
-
Type I interferon related genes are common genes on the early stage after vaccination by meta-analysis of microarray data.Hum Vaccin Immunother. 2015;11(3):739-45. doi: 10.1080/21645515.2015.1008884. Hum Vaccin Immunother. 2015. PMID: 25839220 Free PMC article.
-
Synergistic induction of interferon α through TLR-3 and TLR-9 agonists stimulates immune responses against measles virus in neonatal cotton rats.Vaccine. 2014 Jan 3;32(2):265-70. doi: 10.1016/j.vaccine.2013.11.013. Epub 2013 Nov 18. Vaccine. 2014. PMID: 24262312 Free PMC article.
-
Newcastle Disease Virus as a Vaccine Vector for 20 Years: A Focus on Maternally Derived Antibody Interference.Vaccines (Basel). 2020 May 14;8(2):222. doi: 10.3390/vaccines8020222. Vaccines (Basel). 2020. PMID: 32422944 Free PMC article. Review.
References
-
- Bukreyev, A., and P. L. Collins. 2008. Newcastle disease virus as a vaccine vector for humans. Curr. Opin. Mol. Ther. 10:46-55. - PubMed
-
- Caignard, G., M. Bourai, Y. Jacob, F. Tangy, and P. O. Vidalain. 2009. Inhibition of IFN-alpha/beta signaling by two discrete peptides within measles virus V protein that specifically bind STAT1 and STAT2. Virology 383:112-120. - PubMed
-
- Chang, W. L., E. S. Coro, F. C. Rau, Y. Xiao, D. J. Erle, and N. Baumgarth. 2007. Influenza virus infection causes global respiratory tract B cell response modulation via innate immune signals. J. Immunol. 178:1457-1467. - PubMed
-
- Coro, E. S., W. L. Chang, and N. Baumgarth. 2006. Type I IFN receptor signals directly stimulate local B cells early following influenza virus infection. J. Immunol. 176:4343-4351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

