PB2 residue 158 is a pathogenic determinant of pandemic H1N1 and H5 influenza a viruses in mice
- PMID: 20962098
- PMCID: PMC3014153
- DOI: 10.1128/JVI.01694-10
PB2 residue 158 is a pathogenic determinant of pandemic H1N1 and H5 influenza a viruses in mice
Abstract
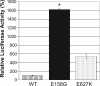
Influenza A viruses are human and animal pathogens that cause morbidity and mortality, which range from mild to severe. The 2009 H1N1 pandemic was caused by the emergence of a reassortant H1N1 subtype (H1N1pdm) influenza A virus containing gene segments that originally circulated in human, avian, and swine virus reservoirs. The molecular determinants of replication and pathogenesis of H1N1pdm viruses in humans and other mammals are poorly understood. Therefore, we set out to elucidate viral determinants critical to the pathogenesis of this novel reassortant using a mouse model. We found that a glutamate-to-glycine substitution at residue 158 of the PB2 gene (PB2-E158G) increased the morbidity and mortality of the parental H1N1pdm virus. Results from mini-genome replication assays in human cells and virus titration in mouse tissues demonstrated that PB2-E158G is a pathogenic determinant, because it significantly increases viral replication rates. The virus load in PB2-E158G-infected mouse lungs was 1,300-fold higher than that of the wild-type virus. Our data also show that PB2-E158G had a much stronger influence on the RNA replication and pathogenesis of H1N1pdm viruses than PB2-E627K, which is a known pathogenic determinant. Remarkably, PB2-E158G substitutions also altered the pathotypes of two avian H5 viruses in mice, indicating that this residue impacts genetically divergent influenza A viruses and suggesting that this region of PB2 could be a new antiviral target. Collectively, the data presented in this study demonstrate that PB2-E158G is a novel pathogenic determinant of influenza A viruses in the mouse model. We speculate that PB2-E158G may be important in the adaptation of avian PB2 genes to other mammals, and BLAST sequence analysis identified a naturally occurring human H1N1pdm isolate that has this substitution. Therefore, future surveillance efforts should include scrutiny of this region of PB2 because of its potential impact on pathogenesis.
Figures

References
-
- Bautista, E., T. Chotpitayasunondh, Z. Gao, S. A. Harper, M. Shaw, T. M. Uyeki, S. R. Zaki, F. G. Hayden, D. S. Hui, J. D. Kettner, A. Kumar, M. Lim, N. Shindo, C. Penn, and K. G. Nicholson. 2010. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N. Engl. J. Med. 362:1708-1719. - PubMed
-
- Fornek, J. L., L. Gillim-Ross, C. Santos, V. Carter, J. M. Ward, L. I. Cheng, S. Proll, M. G. Katze, and K. Subbarao. 2009. A single-amino-acid substitution in a polymerase protein of an H5N1 influenza virus is associated with systemic infection and impaired T-cell activation in mice. J. Virol. 83:11102-11115. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

