Molecular determinants of cardiac fibroblast electrical function and therapeutic implications for atrial fibrillation
- PMID: 20962103
- PMCID: PMC3039247
- DOI: 10.1093/cvr/cvq329
Molecular determinants of cardiac fibroblast electrical function and therapeutic implications for atrial fibrillation
Abstract
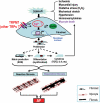
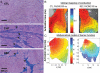
Cardiac fibroblasts account for about 75% of all cardiac cells, but because of their small size contribute only ∼10-15% of total cardiac cell volume. They play a crucial role in cardiac pathophysiology. For a long time, it has been recognized that fibroblasts and related cell types are the principal sources of extracellular matrix (ECM) proteins, which organize cardiac cellular architecture. In disease states, fibroblast production of increased quantities of ECM proteins leads to tissue fibrosis, which can impair both mechanical and electrical function of the heart, contributing to heart failure and arrhythmogenesis. Atrial fibrosis is known to play a particularly important role in atrial fibrillation (AF). This review article focuses on recent advances in understanding the molecular electrophysiology of cardiac fibroblasts. Cardiac fibroblasts express a variety of ion channels, in particular voltage-gated K(+) channels and non-selective cation channels of the transient receptor potential (TRP) family. Both K(+) and TRP channels are important determinants of fibroblast function, with TRP channels acting as Ca(2+)-entry pathways that stimulate fibroblast differentiation into secretory myofibroblast phenotypes producing ECM proteins. Fibroblasts can couple to cardiomyocytes and substantially affect their cellular electrical properties, including conduction, resting potential, repolarization, and excitability. Co-cultured preparations of cardiomyocytes and fibroblasts generate arrhythmias by a variety of mechanisms, including spontaneous impulse formation and rotor-driven reentry. In addition, the excess ECM proteins produced by fibroblasts can interrupt cardiomyocyte-bundle continuity, leading to local conduction disturbances and reentrant arrhythmias. A better understanding of the electrical properties of fibroblasts should lead to an improved comprehension of AF pathophysiology and a variety of novel targets for antiarrhythmic intervention.
Figures


References
-
- Weber K. Cardiac interstitium. In: Poole-Wilson P, Colucci W, Massie B, Chatterjee K, Coats A, editors. Heart Failure. New York, NY: Churchill Livingstone; 1997. pp. p13–31.
-
- Benjamin EJ, Chen P-S, Bild DE, Mascette AM, Albert CM, Alonso A, et al. Prevention of atrial fibrillation: Report from a National Heart, Lung, and Blood Institute Workshop. Circulation. 2009;119:606–618. doi:10.1161/CIRCULATIONAHA.108.825380. - DOI - PMC - PubMed
-
- Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res. 2002;54:230–246. doi:10.1016/S0008-6363(02)00258-4. - DOI - PubMed
-
- Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51:802–809. doi:10.1016/j.jacc.2007.09.064. - DOI - PubMed
-
- Kostin S, Klein G, Szalay Z, Hein S, Bauer EP, Schaper J. Structural correlate of atrial fibrillation in human patients. Cardiovasc Res. 2002;54:361–379. doi:10.1016/S0008-6363(02)00273-0. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

