The contribution of blood lactate to brain energy metabolism in humans measured by dynamic 13C nuclear magnetic resonance spectroscopy
- PMID: 20962220
- PMCID: PMC2996729
- DOI: 10.1523/JNEUROSCI.2040-10.2010
The contribution of blood lactate to brain energy metabolism in humans measured by dynamic 13C nuclear magnetic resonance spectroscopy
Abstract
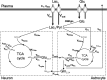
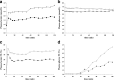
To determine whether plasma lactate can be a significant fuel for human brain energy metabolism, infusions of [3-(13)C]lactate and (1)H-(13)C polarization transfer spectroscopy were used to detect the entry and utilization of lactate. During the 2 h infusion study, (13)C incorporation in the amino acid pools of glutamate and glutamine were measured with a 5 min time resolution. With a plasma concentration ([Lac](P)) being in the 0.8-2.8 mmol/L range, the tissue lactate concentration ([Lac](B)) was assessed as well as the fractional contribution of lactate to brain energy metabolism (CMRlac). From the measured relationship between unidirectional lactate influx (V(in)) and plasma and brain lactate concentrations, lactate transport constants were calculated using a reversible Michaelis-Menten model. The results show that (1) in the physiological range, plasma lactate unidirectional transport (V(in)) and concentration in tissue increase close to linearly with the lactate concentration in plasma; (2) the maximum potential contribution of plasma lactate to brain metabolism is 10% under basal plasma lactate conditions of ∼1.0 mmol/L and as much as 60% at supraphysiological plasma lactate concentrations when the transporters are saturated; (3) the half-saturation constant K(T) is 5.1 ± 2.7 mmol/L and V(MAX) is 0.40 ± 0.13 μmol · g(-1) · min(-1) (68% confidence interval); and (4) the majority of plasma lactate is metabolized in neurons similar to glucose.
Figures






Comment in
-
Lactate transport and metabolism in the human brain: implications for the astrocyte-neuron lactate shuttle hypothesis.J Neurosci. 2011 Mar 30;31(13):4768-70. doi: 10.1523/JNEUROSCI.6612-10.2011. J Neurosci. 2011. PMID: 21451014 Free PMC article. No abstract available.
References
-
- Bachelard H. Landmarks in the application of 13C-magnetic resonance spectroscopy to studies of neuronal/glial relationships. Dev Neurosci. 1998;20:277–288. - PubMed
-
- Blüml S, Moreno-Torres A, Shic F, Nguy CH, Ross BD. Tricarboxylic acid cycle of glia in the in vivo human brain. NMR Biomed. 2002;15:1–5. - PubMed
-
- Chen W, Zhu XH, Gruetter R, Seaquist ER, Adriany G, Uğurbil K. Study of tricarboxylic acid cycle flux changes in human visual cortex during hemifield visual stimulation using 1H-[13C] MRS and fMRI. Magn Reson Med. 2001;45:349–355. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK049230/DK/NIDDK NIH HHS/United States
- P01-DK68229/DK/NIDDK NIH HHS/United States
- K02 AA013430/AA/NIAAA NIH HHS/United States
- R01-AG23686/AG/NIA NIH HHS/United States
- K02-AA13430/AA/NIAAA NIH HHS/United States
- P01 DK068229/DK/NIDDK NIH HHS/United States
- R01 DK040936/DK/NIDDK NIH HHS/United States
- R24 DK085638/DK/NIDDK NIH HHS/United States
- R01 NS037527/NS/NINDS NIH HHS/United States
- M01-RR00125/RR/NCRR NIH HHS/United States
- R01 AG023686/AG/NIA NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- M01 RR000125/RR/NCRR NIH HHS/United States
- R01-NS037527/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
