14-3-3 proteins regulate protein kinase a activity to modulate growth cone turning responses
- PMID: 20962227
- PMCID: PMC6634753
- DOI: 10.1523/JNEUROSCI.3883-10.2010
14-3-3 proteins regulate protein kinase a activity to modulate growth cone turning responses
Abstract
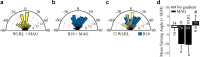
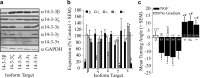
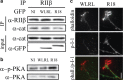
Growth cones regulate the speed and direction of neuronal outgrowth during development and regeneration. How the growth cone spatially and temporally regulates signals from guidance cues is poorly understood. Through a proteomic analysis of purified growth cones we identified isoforms of the 14-3-3 family of adaptor proteins as major constituents of the growth cone. Disruption of 14-3-3 via the R18 antagonist or knockdown of individual 14-3-3 isoforms switches nerve growth factor- and myelin-associated glycoprotein-dependent repulsion to attraction in embryonic day 13 chick and postnatal day 5 rat DRG neurons. These effects are reminiscent of switching responses observed in response to elevated cAMP. Intriguingly, R18-dependent switching is blocked by inhibitors of protein kinase A (PKA), suggesting that 14-3-3 proteins regulate PKA. Consistently, 14-3-3 proteins interact with PKA and R18 activates PKA by dissociating its regulatory and catalytic subunits. Thus, 14-3-3 heterodimers regulate the PKA holoenzyme and this activity plays a critical role in modulating neuronal responses to repellent cues.
Figures




Similar articles
-
Nerve growth factor and semaphorin 3A signaling pathways interact in regulating sensory neuronal growth cone motility.J Neurosci. 2002 Aug 1;22(15):6659-69. doi: 10.1523/JNEUROSCI.22-15-06659.2002. J Neurosci. 2002. PMID: 12151545 Free PMC article.
-
cAMP-dependent axon guidance is distinctly regulated by Epac and protein kinase A.J Neurosci. 2009 Dec 9;29(49):15434-44. doi: 10.1523/JNEUROSCI.3071-09.2009. J Neurosci. 2009. PMID: 20007468 Free PMC article.
-
Growth cones integrate signaling from multiple guidance cues.J Histochem Cytochem. 2003 Apr;51(4):435-44. doi: 10.1177/002215540305100405. J Histochem Cytochem. 2003. PMID: 12642622 Review.
-
The nitric oxide-cGMP pathway controls the directional polarity of growth cone guidance via modulating cytosolic Ca2+ signals.J Neurosci. 2009 Jun 17;29(24):7886-97. doi: 10.1523/JNEUROSCI.0087-09.2009. J Neurosci. 2009. PMID: 19535600 Free PMC article.
-
Switching responses: spatial and temporal regulators of axon guidance.Mol Neurobiol. 2014 Apr;49(2):1077-86. doi: 10.1007/s12035-013-8582-8. Epub 2013 Nov 24. Mol Neurobiol. 2014. PMID: 24271658 Review.
Cited by
-
14-3-3ɛ/ζ Affects the stability of δ-catenin and regulates δ-catenin-induced dendrogenesis.FEBS Open Bio. 2012 Nov 29;3:16-21. doi: 10.1016/j.fob.2012.11.006. Print 2013. FEBS Open Bio. 2012. PMID: 23772369 Free PMC article.
-
14-3-3ζ mediates an alternative, non-thermogenic mechanism in male mice to reduce heat loss and improve cold tolerance.Mol Metab. 2020 Nov;41:101052. doi: 10.1016/j.molmet.2020.101052. Epub 2020 Jul 12. Mol Metab. 2020. PMID: 32668300 Free PMC article.
-
Ribosomes in RNA Granules Are Stalled on mRNA Sequences That Are Consensus Sites for FMRP Association.J Neurosci. 2023 Apr 5;43(14):2440-2459. doi: 10.1523/JNEUROSCI.1002-22.2023. Epub 2023 Feb 27. J Neurosci. 2023. PMID: 36849416 Free PMC article.
-
The dispensability of 14-3-3 proteins for the regulation of human cardiac sodium channel Nav1.5.PLoS One. 2024 Mar 7;19(3):e0298820. doi: 10.1371/journal.pone.0298820. eCollection 2024. PLoS One. 2024. PMID: 38452156 Free PMC article.
-
Pathways to Parkinson's disease: a spotlight on 14-3-3 proteins.NPJ Parkinsons Dis. 2021 Sep 21;7(1):85. doi: 10.1038/s41531-021-00230-6. NPJ Parkinsons Dis. 2021. PMID: 34548498 Free PMC article. Review.
References
-
- Berruti G. A novel rap1/B-Raf/14-3-3 theta protein complex is formed in vivo during the morphogenetic differentiation of postmeiotic male germ cells. Exp Cell Res. 2000;257:172–179. - PubMed
-
- Bridges D, Moorhead GB. 14-3-3 proteins: a number of functions for a numbered protein. Sci STKE. 2005;2005:re10. - PubMed
-
- Clandinin TR, Zipursky SL. Making connections in the fly visual system. Neuron. 2002;35:827–841. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
