Decision time, slow inhibition, and theta rhythm
- PMID: 20962238
- PMCID: PMC6634784
- DOI: 10.1523/JNEUROSCI.0945-10.2010
Decision time, slow inhibition, and theta rhythm
Abstract
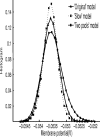
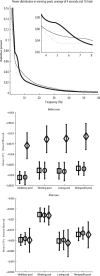
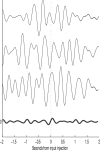
In this paper, we examine decision making in a spiking neuronal network and show that longer time constants for the inhibitory neurons can decrease the reaction times and produce theta rhythm. We analyze the mechanism and find that the spontaneous firing rate before the decision cues are applied can drift, and thereby influence the speed of the reaction time when the decision cues are applied. The drift of the firing rate in the population that will win the competition is larger if the time constant of the inhibitory interneurons is increased from 10 to 33 ms, and even larger if there are two populations of inhibitory neurons with time constants of 10 and 100 ms. Of considerable interest is that the decision that will be made can be influenced by the noise-influenced drift of the spontaneous firing rate over many seconds before the decision cues are applied. The theta rhythm associated with the longer time constant networks mirrors the greater integration in the firing rate drift produced by the recurrent connections over long time periods in the networks with slow inhibition. The mechanism for the effect of slow waves in the theta and delta range on decision times is suggested to be increased neuronal spiking produced by depolarization of the membrane potential on the positive part of the slow waves when the neuron's membrane potential is close to the firing threshold.
Figures




References
-
- Blake R, Logothetis NK. Visual competition. Nat Rev Neurosci. 2002;3:13–21. - PubMed
-
- Boorman ED, Behrens TE, Woolrich MW, Rushworth MF. How green is the grass on the other side? Frontopolar cortex and the evidence in favor of alternative courses of action. Neuron. 2009;62:733–743. - PubMed
-
- Broadbent DE. The magic number seven after fifteen years. In: Kennedy A, Wilkes A, editors. Studies in long term memory. New York: Wiley; 1975. pp. 3–18.
-
- Brunel N, Wang XJ. Effects of neuromodulation in a cortical network model of object working memory dominated by recurrent inhibition. J Comput Neurosci. 2001;11:63–85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
