Experimental characterization and mathematical modeling of P2X7 receptor channel gating
- PMID: 20962242
- PMCID: PMC2980950
- DOI: 10.1523/JNEUROSCI.2390-10.2010
Experimental characterization and mathematical modeling of P2X7 receptor channel gating
Abstract
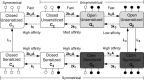
The P2X7 receptor is a trimeric channel with three binding sites for ATP, but how the occupancy of these sites affects gating is still not understood. Here we show that naive receptors activated and deactivated monophasically at low and biphasically at higher agonist concentrations. Both phases of response were abolished by application of Az10606120, a P2X7R-specific antagonist. The slow secondary growth of current in the biphasic response coincided temporally with pore dilation. Repetitive stimulation with the same agonist concentration caused sensitization of receptors, which manifested as a progressive increase in the current amplitude, accompanied by a slower deactivation rate. Once a steady level of the secondary current was reached, responses at high agonist concentrations were no longer biphasic but monophasic. Sensitization of receptors was independent of Na(+) and Ca(2+) influx and ∼30 min washout was needed to reestablish the initial gating properties. T15E- and T15K-P2X7 mutants showed increased sensitivity for agonists, responded with monophasic currents at all agonist concentrations, activated immediately with dilated pores, and deactivated slowly. The complex pattern of gating exhibited by wild-type channels can be accounted for by a Markov state model that includes negative cooperativity of agonist binding to unsensitized receptors caused by the occupancy of one or two binding sites, opening of the channel pore to a low conductance state when two sites are bound, and sensitization with pore dilation to a high conductance state when three sites are occupied.
Figures








References
-
- Becker D, Wolterdorf R, Boldt W, Schmitz S, Braam U, Schmalzing G, Markardt F. The P2X7 carboxyl tail is a regulatory module of P2X7 receptor channel activity. J Biol Chem. 2008;283:25725–25734. - PubMed
-
- Chessell IP, Grahames CBA, Michel AD, Humphrey PPA. Dynamics of P2X7 receptor pore dilation: pharmacological and functional consequences. Drug Dev Res. 2001;53:60–65.
-
- Di Virgilio F, Ceruti S, Bramanti P, Abbracchio MP. Purinergic signalling in inflammation of the central nervous system. Trends Neurosci. 2009b;32:79–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
