Laminar-specific maturation of GABAergic transmission and susceptibility to visual deprivation are related to endocannabinoid sensitivity in mouse visual cortex
- PMID: 20962247
- PMCID: PMC6634750
- DOI: 10.1523/JNEUROSCI.2979-10.2010
Laminar-specific maturation of GABAergic transmission and susceptibility to visual deprivation are related to endocannabinoid sensitivity in mouse visual cortex
Abstract
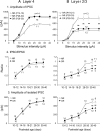
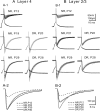
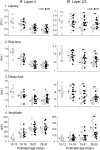
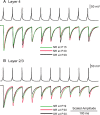
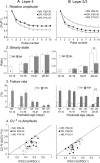
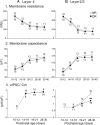
The developmental period when neuronal responses are modified by visual experience is reported to start and end earlier in layer 4 than in layer 2/3 of the visual cortex, and the maturation of GABAergic inhibitory circuits is suggested to determine the timing of this period. Here, we examine whether the laminar difference in such timing corresponds to a difference in the time course of the functional maturation of GABAergic synaptic transmission to star pyramidal and pyramidal cells in layers 4 and 2/3, respectively, of the mouse visual cortex and whether the development of the strength of GABAergic transmission is affected by visual deprivation in a laminar-specific manner. Our analysis of developmental changes in inhibitory postsynaptic currents of star pyramidal and pyramidal cells evoked by electrical stimulation of afferents or action potentials of fast-spiking GABAergic neurons revealed that there was a sequential maturation of GABAergic function from layers 4 to 2/3. The maturation of inhibition in layer 4 occurred at postnatal week 3, which preceded by 1 week that of layer 2/3. Visual deprivation by dark rearing arrested the functional development of GABAergic transmission in layer 2/3, whereas dark rearing was not so effective in layer 4. GABAergic synapses in layer 2/3 were sensitive to an agonist for cannabinoid type 1 receptors and not normally matured in receptor knock-out mice, whereas those in layer 4 were not so. These results suggest laminar-specific maturation of inhibition and susceptibility to visual deprivation, which may be related to the laminar difference in sensitivity to endocannabinoids.
Figures


References
-
- Benevento LA, Bakkum BW, Cohen RS. Gamma-aminobutyric acid and somatostatin immunoreactivity in the visual cortex of normal and dark-reared rats. Brain Res. 1995;689:172–182. - PubMed
-
- Cynader M. Prolonged sensitivity to monocular deprivation in dark-reared cats: effects of age and visual exposure. Brain Res. 1983;284:155–164. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
