Stoichiometry of the KCNQ1 - KCNE1 ion channel complex
- PMID: 20962273
- PMCID: PMC2973890
- DOI: 10.1073/pnas.1010354107
Stoichiometry of the KCNQ1 - KCNE1 ion channel complex
Abstract
The KCNQ1 voltage-gated potassium channel and its auxiliary subunit KCNE1 play a crucial role in the regulation of the heartbeat. The stoichiometry of KCNQ1 and KCNE1 complex has been debated, with some results suggesting that the four KCNQ1 subunits that form the channel associate with two KCNE1 subunits (a 42 stoichiometry), while others have suggested that the stoichiometry may not be fixed. We applied a single molecule fluorescence bleaching method to count subunits in many individual complexes and found that the stoichiometry of the KCNQ1 - KCNE1 complex is flexible, with up to four KCNE1 subunits associating with the four KCNQ1 subunits of the channel (a 44 stoichiometry). The proportion of the various stoichiometries was found to depend on the relative expression densities of KCNQ1 and KCNE1. Strikingly, both the voltage-dependence and kinetics of gating were found to depend on the relative densities of KCNQ1 and KCNE1, suggesting the heart rhythm may be regulated by the relative expression of the auxiliary subunit and the resulting stoichiometry of the channel complex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

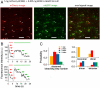
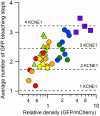

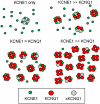
Comment in
-
The cardiac IKs channel, complex indeed.Proc Natl Acad Sci U S A. 2010 Nov 2;107(44):18751-2. doi: 10.1073/pnas.1014150107. Epub 2010 Oct 25. Proc Natl Acad Sci U S A. 2010. PMID: 20974964 Free PMC article. No abstract available.
Similar articles
-
The cardiac IKs channel, complex indeed.Proc Natl Acad Sci U S A. 2010 Nov 2;107(44):18751-2. doi: 10.1073/pnas.1014150107. Epub 2010 Oct 25. Proc Natl Acad Sci U S A. 2010. PMID: 20974964 Free PMC article. No abstract available.
-
The I Ks Ion Channel Activator Mefenamic Acid Requires KCNE1 and Modulates Channel Gating in a Subunit-Dependent Manner.Mol Pharmacol. 2020 Feb;97(2):132-144. doi: 10.1124/mol.119.117952. Epub 2019 Nov 13. Mol Pharmacol. 2020. PMID: 31722973
-
Ginsenoside Rg3 activates human KCNQ1 K+ channel currents through interacting with the K318 and V319 residues: a role of KCNE1 subunit.Eur J Pharmacol. 2010 Jul 10;637(1-3):138-47. doi: 10.1016/j.ejphar.2010.04.001. Epub 2010 Apr 21. Eur J Pharmacol. 2010. PMID: 20399767
-
Insights into Cardiac IKs (KCNQ1/KCNE1) Channels Regulation.Int J Mol Sci. 2020 Dec 11;21(24):9440. doi: 10.3390/ijms21249440. Int J Mol Sci. 2020. PMID: 33322401 Free PMC article. Review.
-
The membrane protein KCNQ1 potassium ion channel: Functional diversity and current structural insights.Biochim Biophys Acta Biomembr. 2020 May 1;1862(5):183148. doi: 10.1016/j.bbamem.2019.183148. Epub 2019 Dec 9. Biochim Biophys Acta Biomembr. 2020. PMID: 31825788 Free PMC article. Review.
Cited by
-
Gating and Regulation of KCNQ1 and KCNQ1 + KCNE1 Channel Complexes.Front Physiol. 2020 Jun 4;11:504. doi: 10.3389/fphys.2020.00504. eCollection 2020. Front Physiol. 2020. PMID: 32581825 Free PMC article. Review.
-
Single-molecule localization to study cytoskeletal structures, membrane complexes, and mechanosensors.Biophys Rev. 2019 Oct;11(5):745-756. doi: 10.1007/s12551-019-00595-2. Epub 2019 Sep 16. Biophys Rev. 2019. PMID: 31529362 Free PMC article. Review.
-
The IKs Channel Response to cAMP Is Modulated by the KCNE1:KCNQ1 Stoichiometry.Biophys J. 2018 Nov 6;115(9):1731-1740. doi: 10.1016/j.bpj.2018.09.018. Epub 2018 Sep 27. Biophys J. 2018. PMID: 30314657 Free PMC article.
-
Detection of a new KCNQ1 frameshift mutation associated with Jervell and Lange-Nielsen syndrome in 2 Iranian families.J Arrhythm. 2018 Apr 16;34(3):286-290. doi: 10.1002/joa3.12042. eCollection 2018 Jun. J Arrhythm. 2018. PMID: 29951145 Free PMC article.
-
Control of Biophysical and Pharmacological Properties of Potassium Channels by Ancillary Subunits.Handb Exp Pharmacol. 2021;267:445-480. doi: 10.1007/164_2021_512. Handb Exp Pharmacol. 2021. PMID: 34247280 Free PMC article.
References
-
- Sanguinetti MC, et al. Coassembly of KVLQT1 and minK (IsK) proteins to form cardiac IKs potassium channel. Nature. 1996;384:80–83. - PubMed
-
- Neyroud N, et al. A novel mutation in the potassium channel gene KVLQT1 causes the Jervell and Lange-Nielsen cardioauditory syndrome. Nat Genet. 1997;15:186–189. - PubMed
-
- Schroeder BC, et al. A constitutively open potassium channel formed by KCNQ1 and KCNE3. Nature. 2000;403:196–199. - PubMed
-
- Long SB, Campbell EB, Mackinnon R. Voltage sensor of Kv1.2: structural basis of electromechanical coupling. Science. 2005;309:903–908. - PubMed
-
- Long SB, Campbell EB, Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

