The road from discovery to clinical diagnostics: lessons learned from the first FDA-cleared in vitro diagnostic multivariate index assay of proteomic biomarkers
- PMID: 20962299
- PMCID: PMC4836873
- DOI: 10.1158/1055-9965.EPI-10-0580
The road from discovery to clinical diagnostics: lessons learned from the first FDA-cleared in vitro diagnostic multivariate index assay of proteomic biomarkers
Abstract
Background: After more than a decade of biomarker discovery research using advanced genomic and proteomic technologies, very few biomarkers have been translated into clinical diagnostics for patient care. This has become an urgent issue to be addressed because the continuing funding from both the public and private sources are called into question.
Methods: We use as an example, OVA1, the first in vitro diagnostic multivariate index assay (IVDMIA) of proteomic biomarkers recently cleared by the US FDA (Food and Drug Administration) to describe our experience through the long road from biomarker discovery, to validation, and finally to multi-institutional trial for regulatory approval by the FDA.
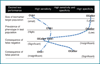
Results: We discuss 3 issues that are key bridges in the path of biomarker development to actual clinical diagnostics: 1) to generate sufficient and "portable" evidence in preliminary validation studies to support investment for large-scale validation trials; 2) to carefully and clearly define clinical utility that balances desire for broad applicability and feasibility for completing clinical trials for regulatory approval; and 3) to select/develop assays with analytical performance suitable for clinical deployment.
Conclusions: We learned that the road from biomarker discovery, validation, to clinical diagnostics could be long and winding, and often frustrating. However, we also know that, with the right approaches, at the end of the road, there is a rainbow waiting for us.
Impact: Provide insights and recommendations for the translation of proteomic biomarkers into clinical diagnostics.
©2010 AACR.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures
References
-
- Chan DW. Will cancer proteomics suffer from premature death? Clin Proteom. 2010;6(1–2):1–3.
-
- Giede KC, Kieser K, Dodge J, Rosen B. Who should operate on patients with ovarian cancer? An evidence-based review. Gynecol Oncol. 2005;99(2):447–461. - PubMed
-
- Zhang Z, Bast RC, Jr, Yu Y, Li J, Sokoll LJ, Rai AJ, et al. Three biomarkers identified from serum proteomic analysis for the detection of early stage ovarian cancer. Cancer Res. 2004;64(16):5882–5890. - PubMed
-
- Rai AJ, Zhang Z, Rosenzweig J, et al. Proteomic approaches to tumor marker discovery – Identification of biomarkers for ovarian cancer. Arch Pathol Med. 2002;126(12):1518–1526. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

