Reversal of depressed behaviors in mice by p11 gene therapy in the nucleus accumbens
- PMID: 20962330
- PMCID: PMC3026098
- DOI: 10.1126/scitranslmed.3001079
Reversal of depressed behaviors in mice by p11 gene therapy in the nucleus accumbens
Erratum in
- Sci Transl Med. 2010 Oct 27;2(55):55er3. Arango-Llievano, Margarita [corrected to Arango-Lievano, Margarita]; Stavarche, Mihaela [corrected to Stavarache, Mihaela]
Abstract
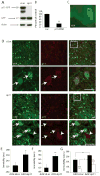
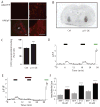
The etiology of major depression remains unknown, but dysfunction of serotonergic signaling has long been implicated in the pathophysiology of this disorder. p11 is an S100 family member recently identified as a serotonin 1B [5-hydroxytryptamine 1B (5-HT(1B))] and serotonin 4 (5-HT(4)) receptor-binding protein. Mutant mice in which p11 is deleted show depression-like behaviors, suggesting that p11 may be a mediator of affective disorder pathophysiology. Using somatic gene transfer, we have now identified the nucleus accumbens as a key site of p11 action. Reduction of p11 with adeno-associated virus (AAV)-mediated RNA interference in the nucleus accumbens, but not in the anterior cingulate, of normal adult mice resulted in depression-like behaviors nearly identical to those seen in p11 knockout mice. Restoration of p11 expression specifically in the nucleus accumbens of p11 knockout mice normalized depression-like behaviors. Human nucleus accumbens tissue shows a significant reduction of p11 protein in depressed patients when compared to matched healthy controls. These results suggest that p11 loss in rodent and human nucleus accumbens may contribute to the pathophysiology of depression. Normalization of p11 expression within this brain region with AAV-mediated gene therapy may be of therapeutic value.
Figures


Comment in
-
p11 and gene therapy for severe psychiatric disorders: a practical goal?Sci Transl Med. 2010 Oct 20;2(54):54ps51. doi: 10.1126/scitranslmed.3001754. Sci Transl Med. 2010. PMID: 20962329
References
-
- Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7:541–547. - PubMed
-
- Svenningsson P, Chergui K, Rachleff I, Flajolet M, Zhang X, El Yacoubi M, Vaugeois JM, Nomikos GG, Greengard P. Alterations in 5-HT1B receptor function by p11 in depression-like states. Science (New York, NY. 2006;311:77–80. - PubMed
-
- Svenningsson P, Greengard P. p11 (S100A10)--an inducible adaptor protein that modulates neuronal functions. Current opinion in pharmacology. 2007;7:27–32. - PubMed
-
- Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J, Hochberg H, Murrough J, Strohmayer E, Stern E, Silbersweig DA. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiatry. 2006;163:1784–1790. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

