Molecular rearrangements involved in the capsid shell maturation of bacteriophage T7
- PMID: 20962334
- PMCID: PMC3012979
- DOI: 10.1074/jbc.M110.187211
Molecular rearrangements involved in the capsid shell maturation of bacteriophage T7
Abstract
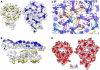
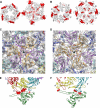
Maturation of dsDNA bacteriophages involves assembling the virus prohead from a limited set of structural components followed by rearrangements required for the stability that is necessary for infecting a host under challenging environmental conditions. Here, we determine the mature capsid structure of T7 at 1 nm resolution by cryo-electron microscopy and compare it with the prohead to reveal the molecular basis of T7 shell maturation. The mature capsid presents an expanded and thinner shell, with a drastic rearrangement of the major protein monomers that increases in their interacting surfaces, in turn resulting in a new bonding lattice. The rearrangements include tilting, in-plane rotation, and radial expansion of the subunits, as well as a relative bending of the A- and P-domains of each subunit. The unique features of this shell transformation, which does not employ the accessory proteins, inserted domains, or molecular interactions observed in other phages, suggest a simple capsid assembling strategy that may have appeared early in the evolution of these viruses.
Figures

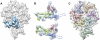
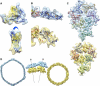
References
-
- Fauquet C. M., Mayo M. A., Maniloff J., Desselberger U., Ball L. A. (eds) (2005) Virus Taxonomy: Eighth Report of the International Committee on Taxonomy of Viruses, Elsevier Academic Press, San Diego
-
- Casjens S., Chiu W., Burnett R. M., Garcea R. L. (1997) in Structural Biology of Viruses, pp. 38–79, Oxford University Press, New York
-
- Valpuesta J. M., Carrascosa J. L. (1994) Q. Rev. Biophys. 27, 107–155 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

