Regional differences in neural crest morphogenesis
- PMID: 20962585
- PMCID: PMC3011260
- DOI: 10.4161/cam.4.4.12890
Regional differences in neural crest morphogenesis
Abstract
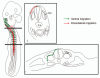
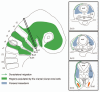
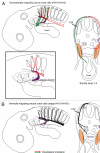
Neural crest cells are pluripotent cells that emerge from the neural epithelium, migrate extensively, and differentiate into numerous derivatives, including neurons, glial cells, pigment cells and connective tissue. Major questions concerning their morphogenesis include: 1) what establishes the pathways of migration and 2) what controls the final destination and differentiation of various neural crest subpopulations. These questions will be addressed in this review. Neural crest cells from the trunk level have been explored most extensively. Studies show that melanoblasts are specified shortly after they depart from the neural tube, and this specification directs their migration into the dorsolateral pathway. We also consider other reports that present strong evidence for ventrally migrating neural crest cells being similarly fate restricted. Cranial neural crest cells have been less analyzed in this regard but the preponderance of evidence indicates that either the cranial neural crest cells are not fate-restricted, or are extremely plastic in their developmental capability and that specification does not control pathfinding. Thus, the guidance mechanisms that control cranial neural crest migration and their behavior vary significantly from the trunk. The vagal neural crest arises at the axial level between the cranial and trunk neural crest and represents a transitional cell population between the head and trunk neural crest. We summarize new data to support this claim. In particular, we show that: 1) the vagal-level neural crest cells exhibit modest developmental bias; 2) there are differences in the migratory behavior between the anterior and the posterior vagal neural crest cells reminiscent of the cranial and the trunk neural crest, respectively; 3) the vagal neural crest cells take the dorsolateral pathway to the pharyngeal arches and the heart, but the ventral pathway to the peripheral nervous system and the gut. However, these pathways are not rigidly specified because of prior fate restriction. Understanding the molecular, cellular and behavioral differences between these three populations of neural crest cells will be of enormous assistance when trying to understand the evolution of the neck.
Figures


Similar articles
-
In the beginning: Generating neural crest cell diversity.Cell Adh Migr. 2010 Oct-Dec;4(4):622-30. doi: 10.4161/cam.4.4.13502. Cell Adh Migr. 2010. PMID: 20930541 Free PMC article. Review.
-
The delayed entry of thoracic neural crest cells into the dorsolateral path is a consequence of the late emigration of melanogenic neural crest cells from the neural tube.Dev Biol. 1998 Aug 15;200(2):234-46. doi: 10.1006/dbio.1998.8963. Dev Biol. 1998. PMID: 9705230
-
Vagal neural crest cell migratory behavior: a transition between the cranial and trunk crest.Dev Dyn. 2011 Sep;240(9):2084-100. doi: 10.1002/dvdy.22715. Dev Dyn. 2011. PMID: 22016183 Free PMC article.
-
Specification and migration of melanoblasts at the vagal level and in hyperpigmented Silkie chickens.Dev Dyn. 1998 Dec;213(4):476-85. doi: 10.1002/(SICI)1097-0177(199812)213:4<476::AID-AJA12>3.0.CO;2-R. Dev Dyn. 1998. PMID: 9853968
-
Neural crest development: the interplay between morphogenesis and cell differentiation.Curr Top Dev Biol. 1998;40:177-209. doi: 10.1016/s0070-2153(08)60367-1. Curr Top Dev Biol. 1998. PMID: 9673851 Review.
Cited by
-
Development of the Autonomic Nervous System: Clinical Implications.Semin Neurol. 2020 Oct;40(5):473-484. doi: 10.1055/s-0040-1713926. Epub 2020 Sep 14. Semin Neurol. 2020. PMID: 32927484 Free PMC article. Review.
-
Embryonic Exposure to Cigarette Smoke Extract Impedes Skeletal Development and Evokes Craniofacial Defects in Zebrafish.Int J Mol Sci. 2022 Aug 31;23(17):9904. doi: 10.3390/ijms23179904. Int J Mol Sci. 2022. PMID: 36077301 Free PMC article.
-
Chicken trunk neural crest migration visualized with HNK1.Acta Histochem. 2015 Apr;117(3):255-66. doi: 10.1016/j.acthis.2015.03.002. Epub 2015 Mar 21. Acta Histochem. 2015. PMID: 25805416 Free PMC article.
-
Quadruple genetic variants in a sporadic ALS patient.Mol Genet Genomic Med. 2022 Jul;10(7):e1953. doi: 10.1002/mgg3.1953. Epub 2022 Apr 14. Mol Genet Genomic Med. 2022. PMID: 35426263 Free PMC article.
-
Reprogramming of trunk neural crest to a cranial crest-like identity alters their transcriptome and developmental potential.Differentiation. 2023 May-Jun;131:27-37. doi: 10.1016/j.diff.2023.04.001. Epub 2023 Apr 5. Differentiation. 2023. PMID: 37058884 Free PMC article.
References
-
- Harris ML, Erickson CA. Lineage specification in neural crest cell pathfinding. Dev Dyn. 2007;236:1–19. - PubMed
-
- Reedy MV, Faraco CD, Erickson CA. The delayed entry of thoracic neural crest cells into the dorsolateral path is a consequence of the late emigration of melanogenic neural crest cells from the neural tube. Dev Biol. 1998;200:234–246. - PubMed
-
- Henion PD, Weston JA. Timing and pattern of cell fate restrictions in the neural crest lineage. Development. 1997;124:4351–4359. - PubMed
-
- Wakamatsu Y, Mochii M, Vogel KS, Weston JA. Avian neural crest-derived neurogenic precursors undergo apoptosis on the lateral migration pathway. Development. 1998;125:4205–4213. - PubMed
-
- Richardson MK, Sieber-Blum M. Pluripotent neural crest cells in the developing skin of the quail embryo. Dev Biol. 1993;157:348–358. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
