Autoimmunity in Coxsackievirus B3 induced myocarditis: role of estrogen in suppressing autoimmunity
- PMID: 20963181
- PMCID: PMC2956018
- DOI: 10.2217/fvl.10.19
Autoimmunity in Coxsackievirus B3 induced myocarditis: role of estrogen in suppressing autoimmunity
Abstract
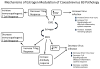
Picornaviruses are small, non-enveloped, single stranded, positive sense RNA viruses which cause multiple diseases including myocarditis/dilated cardiomyopathy, type 1 diabetes, encephalitis, myositis, orchitis and hepatitis. Although picornaviruses directly kill cells, tissue injury primarily results from autoimmunity to self antigens. Viruses induce autoimmunity by: aborting deletion of self-reactive T cells during T cell ontogeny; reversing anergy of peripheral autoimmune T cells; eliminating T regulatory cells; stimulating self-reactive T cells through antigenic mimicry or cryptic epitopes; and acting as an adjuvant for self molecules released during virus infection. Most autoimmune diseases (SLE, rheumatoid arthritis, Grave's disease) predominate in females, but diseases associated with picornavirus infections predominate in males. T regulatory cells are activated in infected females because of the combined effects of estrogen and innate immunity.
Figures
Similar articles
-
Autoimmunity in coxsackievirus B3 induced myocarditis.Autoimmunity. 2006 Feb;39(1):55-61. doi: 10.1080/08916930500484906. Autoimmunity. 2006. PMID: 16455582 Review.
-
Autoimmunity in picornavirus infections.Curr Opin Virol. 2016 Feb;16:8-14. doi: 10.1016/j.coviro.2015.10.004. Epub 2015 Nov 8. Curr Opin Virol. 2016. PMID: 26554915 Free PMC article. Review.
-
Dual functions of murine gammadelta cells in inflammation and autoimmunity in coxsackievirus B3-induced myocarditis: role of Vgamma1+ and Vgamma4+ cells.Microbes Infect. 2005 Mar;7(3):537-43. doi: 10.1016/j.micinf.2004.12.011. Epub 2005 Feb 5. Microbes Infect. 2005. PMID: 15777711 Review.
-
Potential clinical implications of molecular mimicry-induced autoimmunity.Immun Inflamm Dis. 2024 Feb;12(2):e1178. doi: 10.1002/iid3.1178. Immun Inflamm Dis. 2024. PMID: 38415936 Free PMC article. Review.
-
Virus-induced autoimmunity: epitope spreading to myelin autoepitopes in Theiler's virus infection of the central nervous system.Adv Virus Res. 2001;56:199-217. doi: 10.1016/s0065-3527(01)56008-x. Adv Virus Res. 2001. PMID: 11450300 Review.
Cited by
-
The role of sex differences in autophagy in the heart during coxsackievirus B3-induced myocarditis.J Cardiovasc Transl Res. 2014 Mar;7(2):182-91. doi: 10.1007/s12265-013-9525-5. Epub 2013 Dec 10. J Cardiovasc Transl Res. 2014. PMID: 24323874 Free PMC article. Review.
References
-
- Goldberg E, Krause I. Infection and type 1 diabetes mellitus - a two edged sword? Autoimmunity reviews. 2009;8(8):682–686. - PubMed
-
- Root-Bernstein R, Vonck J, Podufaly A. Antigenic complementarity between coxsackie virus and streptococcus in the induction of rheumatic heart disease and autoimmune myocarditis. Autoimmunity. 2009;42(1):1–16. - PubMed
-
- Fairweather D, Kaya Z, Shellam Gr, Lawson Cm, Rose Nr. From infection to autoimmunity. J Autoimmun. 2001;16(3):175–186. - PubMed
-
- Pohl D. Epstein-barr virus and multiple sclerosis. J Neurol Sci. 2009;286(1–2):62–64. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
