Pitfalls of vaccinations with WT1-, Proteinase3- and MUC1-derived peptides in combination with MontanideISA51 and CpG7909
- PMID: 20963411
- PMCID: PMC3024516
- DOI: 10.1007/s00262-010-0929-7
Pitfalls of vaccinations with WT1-, Proteinase3- and MUC1-derived peptides in combination with MontanideISA51 and CpG7909
Abstract
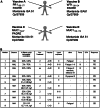
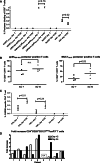
T cells with specificity for antigens derived from Wilms Tumor gene (WT1), Proteinase3 (Pr3), and mucin1 (MUC1) have been demonstrated to lyse acute myeloid leukemia (AML) blasts and multiple-myeloma (MM) cells, and strategies to enhance or induce such tumor-specific T cells by vaccination are currently being explored in multiple clinical trials. To test safety and immunogenicity of a vaccine composed of WT1-, Pr3-, and MUC1-derived Class I-restricted peptides and the pan HLA-DR T helper cell epitope (PADRE) or MUC1-helper epitopes in combination with CpG7909 and MontanideISA51, four patients with AML and five with MM were repetitively vaccinated. No clinical responses were observed. Neither pre-existing nor naive WT1-/Pr3-/MUC1-specific CD8+ T cells expanded in vivo by vaccination. In contrast, a significant decline in vaccine-specific CD8+ T cells was observed. An increase in PADRE-specific CD4+ T helper cells was observed after vaccination but these appeared unable to produce IL2, and CD4+ T cells with a regulatory phenotype increased. Taken into considerations that multiple clinical trials with identical antigens but different adjuvants induced vaccine-specific T cell responses, our data caution that a vaccination with leukemia-associated antigens can be detrimental when combined with MontanideISA51 and CpG7909. Reflecting the time-consuming efforts of clinical trials and the fact that 1/3 of ongoing peptide vaccination trails use CpG and/or Montanide, our data need to be taken into consideration.
Figures
References
-
- Molldrem J, Dermime S, Parker K, Jiang YZ, Mavroudis D, Hensel N, Fukushima P, Barrett AJ. Targeted T-cell therapy for human leukemia: cytotoxic T lymphocytes specific for a peptide derived from proteinase 3 preferentially lyse human myeloid leukemia cells. Blood. 1996;88:2450–2457. - PubMed
-
- Molldrem JJ, Clave E, Jiang YZ, Mavroudis D, Raptis A, Hensel N, Agarwala V, Barrett AJ. Cytotoxic T lymphocytes specific for a nonpolymorphic proteinase 3 peptide preferentially inhibit chronic myeloid leukemia colony-forming units. Blood. 1997;90:2529–2534. - PubMed
-
- Wolfl M, Kuball J, Ho WY, Nguyen H, Manley TJ, Bleakley M, Greenberg PD. Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood. 2007;110:201–210. doi: 10.1182/blood-2006-11-056168. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

