Randomized trial of biofilm testing to select antibiotics for cystic fibrosis airway infection
- PMID: 20963843
- PMCID: PMC3479399
- DOI: 10.1002/ppul.21350
Randomized trial of biofilm testing to select antibiotics for cystic fibrosis airway infection
Abstract
Rationale: In cystic fibrosis (CF), conventional antibiotic susceptibility results correlate poorly with clinical outcomes. We hypothesized that biofilm testing would more accurately reflect the susceptibilities of bacteria infecting CF airways.
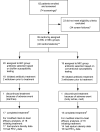
Methods: A multicenter randomized pilot trial was conducted to assess the efficacy and safety of using biofilm susceptibility testing of Pseudomonas aeruginosa sputum isolates to guide antibiotic regimens for chronic airway infections in clinically stable adolescent and adult CF patients. Thirty-nine participants were randomized to biofilm or conventional treatment groups; 14-day courses of two antibiotics were selected according to an activity-based algorithm using the corresponding susceptibility results.
Results: Of the agents tested, meropenem was most active against biofilm-grown bacteria, and was included in regimens for about half of each study group. For 19 of 39 randomized participants, randomization to the other study group would not have changed the antibiotic classes of the assigned regimen. Study groups were comparable at baseline, and had similar mean decreases in bacterial density, measured in log(10) colony forming units per gram of sputum (biofilm, -2.94 [SD 2.83] vs. conventional, -3.27 [SD 3.09]), and mean increases in forced expiratory volume in 1 sec, measured in liters (0.18 [SD 0.20] vs. 0.12 [SD 0.22]).
Conclusions: In this pilot study, antibiotic regimens based on biofilm testing did not differ significantly from regimens based on conventional testing in terms of microbiological and clinical responses. The predictive value of biofilm testing may nonetheless warrant evaluation in an adequately powered clinical trial in younger CF patients or those experiencing acute pulmonary exacerbation.
Copyright © 2011 Wiley-Liss, Inc.
Figures
References
-
- Cystic Fibrosis Foundation . Patient Registry Annual Data Report 2006. Bethesda, Maryland: 2008. CFF Patient Registry.
-
- Moskowitz SM, Silva SJ, Mayer-Hamblett N, Pasta DJ, Mink DR, Mabie JA, Konstan MW, Wagener JS. Shifting patterns of inhaled antibiotic use in cystic fibrosis. Pediatr Pulmonol. 2008;43:874–881. - PubMed
-
- Burns JL, Emerson J, Stapp JR, Yim DL, Krzewinski J, Louden L, Ramsey BW, Clausen CR. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin Infect Dis. 1998;27:158–163. - PubMed
-
- Smith AL, Doershuk C, Goldmann D, Gore E, Hilman B, Marks M, Moss R, Ramsey B, Redding G, Rubio T, Williams-Warren J, Wilmott R, Wilson HD, Yogev R. Comparison of a beta-lactam alone versus beta-lactam and an aminoglycoside for pulmonary exacerbation in cystic fibrosis. J Pediatr. 1999;134:413–421. - PubMed
-
- Emerson J, McNamara S, Buccat AM, Worrell K, Burns JL. Changes in cystic fibrosis sputum microbiology in the United States between 1995 and 2008. Pediatr Pulmonol. 2010;45:363–370. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- M01 RR000034/RR/NCRR NIH HHS/United States
- M01 RR000036/RR/NCRR NIH HHS/United States
- M01 RR000188/RR/NCRR NIH HHS/United States
- M01 RR000037/RR/NCRR NIH HHS/United States
- M01 RR000059/RR/NCRR NIH HHS/United States
- UL1RR025014/RR/NCRR NIH HHS/United States
- UL1 RR025014/RR/NCRR NIH HHS/United States
- UL1 RR025755/RR/NCRR NIH HHS/United States
- M01 RR000084/RR/NCRR NIH HHS/United States
- UL1 RR024979/RR/NCRR NIH HHS/United States
- K08 HL067903/HL/NHLBI NIH HHS/United States
- UL1 RR024153/RR/NCRR NIH HHS/United States
- M01 RR008084/RR/NCRR NIH HHS/United States
- UL1RR024992/RR/NCRR NIH HHS/United States
- UL1 RR024992/RR/NCRR NIH HHS/United States
- UL1 RR026314/RR/NCRR NIH HHS/United States
- K08HL067903/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

