Safety and maximum tolerated dose of superselective intraarterial cerebral infusion of bevacizumab after osmotic blood-brain barrier disruption for recurrent malignant glioma. Clinical article
- PMID: 20964595
- PMCID: PMC3622705
- DOI: 10.3171/2010.9.JNS101223
Safety and maximum tolerated dose of superselective intraarterial cerebral infusion of bevacizumab after osmotic blood-brain barrier disruption for recurrent malignant glioma. Clinical article
Abstract
Object: The authors assessed the safety and maximum tolerated dose of superselective intraarterial cerebral infusion (SIACI) of bevacizumab after osmotic disruption of the blood-brain barrier (BBB) with mannitol in patients with recurrent malignant glioma.
Methods: A total of 30 patients with recurrent malignant glioma were included in the current study.
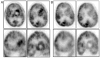
Results: The authors report no dose-limiting toxicity from a single dose of SIACI of bevacizumab up to 15 mg/kg after osmotic BBB disruption with mannitol. Two groups of patients were studied; those without prior bevacizumab exposure (naïve patients; Group I) and those who had received previous intravenous bevacizumab (exposed patients; Group II). Radiographic changes demonstrated on MR imaging were assessed at 1 month postprocedure. In Group I patients, MR imaging at 1 month showed a median reduction in the area of tumor enhancement of 34.7%, a median reduction in the volume of tumor enhancement of 46.9%, a median MR perfusion (MRP) reduction of 32.14%, and a T2-weighted/FLAIR signal decrease in 9 (47.4%) of 19 patients. In Group II patients, MR imaging at 1 month showed a median reduction in the area of tumor enhancement of 15.2%, a median volume reduction of 8.3%, a median MRP reduction of 25.5%, and a T2-weighted FLAIR decrease in 0 (0%) of 11 patients.
Conclusions: The authors conclude that SIACI of mannitol followed by bevacizumab (up to 15 mg/kg) for recurrent malignant glioma is safe and well tolerated. Magnetic resonance imaging shows that SIACI treatment with bevacizumab can lead to reduction in tumor area, volume, perfusion, and T2-weighted/FLAIR signal.
Figures



References
-
- Abrey LE. Bevacizumab in recurrent malignant glioma. Curr Neurol Neurosci Rep. 2008;8:233–234. - PubMed
-
- Bullard DE, Bigner DD. Blood-brain barrier disruption in immature Fischer 344 rats. J Neurosurg. 1984;60:743–750. - PubMed
-
- Bullard DE, Bigner SH, Bigner DD. Comparison of intravenous versus intracarotid therapy with 1,3-bis(2-chloroethyl)-1-nitrosourea in a rat brain tumor model. Cancer Res. 1985;45:5240–5245. - PubMed
-
- Cloughesy TF, Black KL, Gobin YP, Farahani K, Nelson G, Villablanca P, et al. Intra-arterial Cereport (RMP-7) and carboplatin: a dose escalation study for recurrent malignant gliomas. Neurosurgery. 1999;44:270–279. - PubMed
-
- Cloughesy TF, Gobin YP, Black KL, Viñuela F, Taft F, Kadkhoda B, et al. Intra-arterial carboplatin chemotherapy for brain tumors: a dose escalation study based on cerebral blood flow. J Neurooncol. 1997;35:121–131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

