Polarized targeting of L1-CAM regulates axonal and dendritic bundling in vitro
- PMID: 20964729
- PMCID: PMC2981701
- DOI: 10.1111/j.1460-9568.2010.07447.x
Polarized targeting of L1-CAM regulates axonal and dendritic bundling in vitro
Abstract
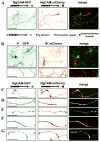
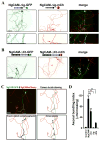
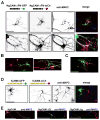
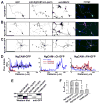
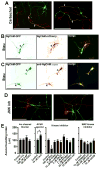
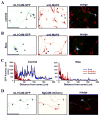
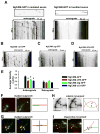
Proper axonal and dendritic bundling is essential for the establishment of neuronal connections and the synchronization of synaptic inputs, respectively. Cell adhesion molecules of the L1-CAM (L1-cell adhesion molecule) family regulate axon guidance and fasciculation, neuron migration, dendrite morphology, and synaptic plasticity. It remains unclear how these molecules play so many different roles. Here we show that polarized axon-dendrite targeting of an avian L1-CAM protein, NgCAM (neuron-glia cell adhesion molecule), can regulate the switch of bundling of the two major compartments of rat hippocampal neurons. Using a new in-vitro model for studying neurite-neurite interactions, we found that expressed axonal NgCAM induced robust axonal bundling via the trans-homophilic interaction of immunoglobulin domains. Interestingly, dendritic bundling was induced by the dendritic targeting of NgCAM, caused by either deleting its fibronectin repeats or blocking activities of protein kinases. Consistent with the NgCAM results, expression of mouse L1-CAM also induced axonal bundling and blocking kinase activities disrupted its axonal targeting. Furthermore, the trans-homophilic interaction stabilized the bundle formation, probably through recruiting NgCAM proteins to contact sites and promoting guided axon outgrowth. Taken together, our results suggest that precise localization of L1-CAM is important for establishing proper cell-cell contacts in neural circuits.
© 2010 The Authors. European Journal of Neuroscience © 2010 Federation of European Neuroscience Societies and Blackwell Publishing Ltd.
Figures
References
-
- Bak M, Fraser SE. Axon fasciculation and differences in midline kinetics between pioneer and follower axons within commissural fascicles. Development. 2003;130:4999–5008. - PubMed
-
- Bennett V, Chen L. Ankyrins and cellular targeting of diverse membrane proteins to physiological sites. Curr Opin Cell Biol. 2001;13:61–67. - PubMed
-
- Cohen NR, Taylor JS, Scott LB, Guillery RW, Soriano P, Furley AJ. Errors in corticospinal axon guidance in mice lacking the neural cell adhesion molecule L1. Curr Biol. 1998;8:26–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

