Toll-like receptor 4 signaling in liver injury and hepatic fibrogenesis
- PMID: 20964825
- PMCID: PMC2984459
- DOI: 10.1186/1755-1536-3-21
Toll-like receptor 4 signaling in liver injury and hepatic fibrogenesis
Abstract
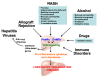
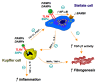
Toll-like receptors (TLRs) are a family of transmembrane pattern recognition receptors (PRR) that play a key role in innate and adaptive immunity by recognizing structural components unique to bacteria, fungi and viruses. TLR4 is the most studied of the TLRs, and its primary exogenous ligand is lipopolysaccharide, a component of Gram-negative bacterial walls. In the absence of exogenous microbes, endogenous ligands including damage-associated molecular pattern molecules from damaged matrix and injured cells can also activate TLR4 signaling. In humans, single nucleotide polymorphisms of the TLR4 gene have an effect on its signal transduction and on associated risks of specific diseases, including cirrhosis. In liver, TLR4 is expressed by all parenchymal and non-parenchymal cell types, and contributes to tissue damage caused by a variety of etiologies. Intact TLR4 signaling was identified in hepatic stellate cells (HSCs), the major fibrogenic cell type in injured liver, and mediates key responses including an inflammatory phenotype, fibrogenesis and anti-apoptotic properties. Further clarification of the function and endogenous ligands of TLR4 signaling in HSCs and other liver cells could uncover novel mechanisms of fibrogenesis and facilitate the development of therapeutic strategies.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources

