Oophorectomy, menopause, estrogen treatment, and cognitive aging: clinical evidence for a window of opportunity
- PMID: 20965156
- PMCID: PMC3046246
- DOI: 10.1016/j.brainres.2010.10.031
Oophorectomy, menopause, estrogen treatment, and cognitive aging: clinical evidence for a window of opportunity
Abstract
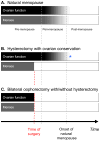
The neuroprotective effects of estrogen have been demonstrated consistently in cellular and animal studies but the evidence in women remains conflicted. We explored the window of opportunity hypothesis in relation to cognitive aging and dementia. In particular, we reviewed existing literature, reanalyzed some of our data, and combined results graphically. Current evidence suggests that estrogen may have beneficial, neutral, or detrimental effects on the brain depending on age at the time of treatment, type of menopause (natural versus medically or surgically induced), or stage of menopause. The comparison of women who underwent bilateral oophorectomy with referent women provided evidence for a sizeable neuroprotective effect of estrogen before age 50 years. Several case-control studies and cohort studies also showed neuroprotective effects in women who received estrogen treatment (ET) in the early postmenopausal stage (most commonly at ages 50-60 years). The majority of women in those observational studies had undergone natural menopause and were treated for the relief of menopausal symptoms. However, recent clinical trials by the Women's Health Initiative showed that women who initiated ET alone or in combination with a progestin in the late postmenopausal stage (ages 65-79 years) experienced an increased risk of dementia and cognitive decline regardless of the type of menopause. The current conflicting data can be explained by the window of opportunity hypothesis suggesting that the neuroprotective effects of estrogen depend on age at the time of administration, type of menopause, and stage of menopause. Therefore, women who underwent bilateral oophorectomy before the onset of menopause or women who experienced premature or early natural menopause should be considered for hormonal treatment until approximately age 51 years.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures


References
-
- Allison MA, Manson JE, Aragaki A, Langer RD, Rossouw J, Curb D, Martin LW, Phillips L, Stefanick ML, Cochrane BB, Sarto G, Barnhart J, O’Sullivan MJ, Johnson KC, Gass M, Trevisan M, Woods NF. Vasomotor symptoms and coronary artery calcium in postmenopausal women. Menopause. 2010;17 Epub ahead of print. - PMC - PubMed
-
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 89 Elective and risk-reducing salpingo-oophorectomy. Obstet Gynecol. 2008;111:231–241. - PubMed
-
- Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O’Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. - PubMed
-
- Armstrong K, Schwartz JS, Randall T, Rubin SC, Weber B. Hormone replacement therapy and life expectancy after prophylactic oophorectomy in women with BRCA1/2 mutations: a decision analysis. J Clin Oncol. 2004;22:1045–1054. - PubMed
-
- Banks E, Canfell K. Invited Commentary: Hormone therapy risks and benefits--The Women’s Health Initiative findings and the postmenopausal estrogen timing hypothesis. Am J Epidemiol. 2009;170:24–28. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

