Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery: a randomized controlled trial
- PMID: 20966692
- PMCID: PMC3104850
- DOI: 10.1097/AOG.0b013e3181f73fac
Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery: a randomized controlled trial
Abstract
Objective: To estimate whether 6-month use of the levonorgestrel-releasing intrauterine device (IUD) would be higher when insertion occurred within 10 minutes of placental delivery compared with 6-8 weeks postpartum.
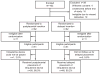
Methods: We enrolled pregnant women planning vaginal deliveries and desiring a postpartum levonorgestrel-releasing IUD. Patients were randomly assigned when admitted in labor to postplacental or delayed IUD insertion. The women followed up in person at 6-8 weeks and 6 months and were contacted by telephone at 3 months. Women were ineligible for a study IUD postenrollment for intrapartum events including infection, hemorrhage, and cesarean delivery; these women were contacted by phone at 3 and 6 months. Expelled IUDs were replaced per patient preference.
Results: Successful IUD placement occurred in 50 of 51 participants (98.0%) and 46 of 51 participants (90.2%) in the postplacental and delayed groups, respectively (P=.2). Expulsion within 6 months occurred in 12 of 50 (24.0%; 95% confidence interval [CI], 13.1-38.2) and two of 46 (4.4%; 95% CI 0.5-14.8) participants, respectively (P=.008). Intrauterine device use at 6 months was 43 of 51 (84.3%; 95% CI 71.4-93.0) and 39 of 51 (76.5%; 95% CI 62.5-87.2), respectively (P=.32). For ineligible patients, only 11 of 41 (26.8%) women were using IUDs at 6 months and two (4.9%) had become pregnant.
Conclusion: Intrauterine device use 6 months after delivery is similar in women who have postpartum or scheduled delayed IUD placement through a study after replacement of expelled IUDs. Expulsions are significantly higher with postplacental compared with delayed IUD placement. Women asked to follow up with their own health care providers for delayed insertion are significantly less likely to receive an IUD.
Clinical trial registration: ClinicalTrials.gov, www.clinicaltrials.gov, NCT00476021.
Level of evidence: I.
Figures

References
-
- Finer LB, Henshaw SK. Disparities in rates of unintended pregnancy in the United States, 1994 and 2001. Perspect Sex Reprod Health. 2006;38:90–6. - PubMed
-
- Kershaw TS, Niccolai LM, Ickovics JR, Lewis JB, Meade CS, Ethier KA. Short and long-term impact of adolescent pregnancy on postpartum contraceptive use: implications for prevention of repeat pregnancy. J Adolesc Health. 2003;33:359–68. - PubMed
-
- Templeman CL, Cook V, Goldsmith LJ, Powell J, Hertweck SP. Postpartum contraceptive use among adolescent mothers. Obstet Gynecol. 2000;95:770–6. - PubMed
-
- Shulman JJ, Merritt CG. Postpartum contraception: subsequent pregnancy, delivery, and abortion rates. Fertil Steril. 1976;27:97–103. - PubMed
-
- Ogburn JA, Espey E, Stonehocker J. Barriers to intrauterine device insertion in postpartum women. Contraception. 2005;72:426–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

