Chronic optical access through a polished and reinforced thinned skull
- PMID: 20966916
- PMCID: PMC3204312
- DOI: 10.1038/nmeth.1530
Chronic optical access through a polished and reinforced thinned skull
Abstract
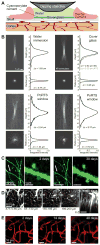
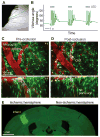
We present a method to form an optical window in the mouse skull that spans millimeters and is stable for months without causing brain inflammation. This enabled us to repeatedly image blood flow in cortical capillaries of awake mice and determine long-range correlations in speed. We also repeatedly imaged dendritic spines, microglia and angioarchitecture, as well as used illumination to drive motor output via optogenetics and induce microstrokes via photosensitizers.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Svoboda K, Denk W, Kleinfeld D, Tank DW. Nature. 1997;385:161. - PubMed
-
- Levasseur JE, Wei EP, Raper AJ, Kontos AA, Patterson JL. Stroke. 1975;6:308. - PubMed
-
- Grutzendler J, Kasthuri N, Gan WB. Nature. 2002;420:812. - PubMed
-
- Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, Svoboda K. Nature. 2002;420:788. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RR021907/RR/NCRR NIH HHS/United States
- R01 NS066361/NS/NINDS NIH HHS/United States
- R01 EB003832/EB/NIBIB NIH HHS/United States
- R01 NS052189/NS/NINDS NIH HHS/United States
- DP1 NS082097/NS/NINDS NIH HHS/United States
- NS066361/NS/NINDS NIH HHS/United States
- MH085499/MH/NIMH NIH HHS/United States
- R01 MH085499/MH/NIMH NIH HHS/United States
- R21 RR021907/RR/NCRR NIH HHS/United States
- NS059832/NS/NINDS NIH HHS/United States
- DP1 OD006831/OD/NIH HHS/United States
- EB003832/EB/NIBIB NIH HHS/United States
- R21 NS059832/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

