Recognition-mediated activation of therapeutic gold nanoparticles inside living cells
- PMID: 20966953
- PMCID: PMC2967735
- DOI: 10.1038/nchem.858
Recognition-mediated activation of therapeutic gold nanoparticles inside living cells
Abstract
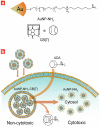
Supramolecular chemistry provides a versatile tool for the organization of molecular systems into functional structures and the actuation of these assemblies for applications through the reversible association between complementary components. Use of this methodology in living systems, however, represents a significant challenge owing to the chemical complexity of cellular environments and lack of selectivity of conventional supramolecular interactions. Herein, we present a host-guest system featuring diaminohexane-terminated gold nanoparticles (AuNP-NH(2)) and complementary cucurbit[7]uril (CB[7]). In this system, threading of CB[7] on the particle surface reduces the cytotoxicity of AuNP-NH(2) through sequestration of the particle in endosomes. Intracellular triggering of the therapeutic effect of AuNP-NH(2) was then achieved through the administration of 1-adamantylamine (ADA), removing CB[7] from the nanoparticle surface, causing the endosomal release and concomitant in situ cytotoxicity of AuNP-NH(2). This supramolecular strategy for intracellular activation provides a new tool for potential therapeutic applications.
Figures




References
-
- Lehn JM. Toward self-organization and complex matter. Science. 2002;295:2400–2403. - PubMed
-
- Lehn JM. From supramolecular chemistry towards constitutional dynamic chemistry and adaptive chemistry. Chem. Soc. Rev. 2007;36:151–160. - PubMed
-
- Reinhoudt DN, Crego-Calama M. Synthesis beyond the molecule. Science. 2002;295:2403–2407. - PubMed
-
- Yaghi OM, et al. Reticular synthesis and the design of new materials. Nature. 2003;423:705–714. - PubMed
-
- Lehn JM. Supramolecular Chemistry: Concepts and Perspectives. VCH; New York: 1995.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

