Emergence of noise-induced oscillations in the central circadian pacemaker
- PMID: 20967239
- PMCID: PMC2953532
- DOI: 10.1371/journal.pbio.1000513
Emergence of noise-induced oscillations in the central circadian pacemaker
Erratum in
- PLoS Biol. 2010;8(10) doi: 10.1371/annotation/45e0bb8b-69bd-4ba0-bb9e-6641bce10fc6.
Abstract
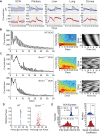
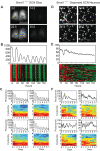
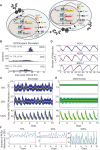
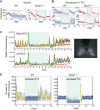
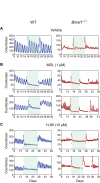
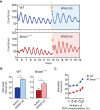
Bmal1 is an essential transcriptional activator within the mammalian circadian clock. We report here that the suprachiasmatic nucleus (SCN) of Bmal1-null mutant mice, unexpectedly, generates stochastic oscillations with periods that overlap the circadian range. Dissociated SCN neurons expressed fluctuating levels of PER2 detected by bioluminescence imaging but could not generate circadian oscillations intrinsically. Inhibition of intercellular communication or cyclic-AMP signaling in SCN slices, which provide a positive feed-forward signal to drive the intracellular negative feedback loop, abolished the stochastic oscillations. Propagation of this feed-forward signal between SCN neurons then promotes quasi-circadian oscillations that arise as an emergent property of the SCN network. Experimental analysis and mathematical modeling argue that both intercellular coupling and molecular noise are required for the stochastic rhythms, providing a novel biological example of noise-induced oscillations. The emergence of stochastic circadian oscillations from the SCN network in the absence of cell-autonomous circadian oscillatory function highlights a previously unrecognized level of circadian organization.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Welsh D. K, Logothetis D. E, Meister M, Reppert S. M. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. - PubMed
-
- Nagoshi E, Saini C, Bauer C, Laroche T, Naef F, et al. Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell. 2004;119:693–705. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

