Rapid and highly sensitive detection of malaria-infected erythrocytes using a cell microarray chip
- PMID: 20967248
- PMCID: PMC2954146
- DOI: 10.1371/journal.pone.0013179
Rapid and highly sensitive detection of malaria-infected erythrocytes using a cell microarray chip
Abstract
Background: Malaria is one of the major human infectious diseases in many endemic countries. For prevention of the spread of malaria, it is necessary to develop an early, sensitive, accurate and conventional diagnosis system.
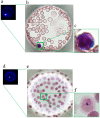
Methods and findings: A cell microarray chip was used to detect for malaria-infected erythrocytes. The chip, with 20,944 microchambers (105 µm width and 50 µm depth), was made from polystyrene, and the formation of monolayers of erythrocytes in the microchambers was observed. Cultured Plasmodium falciparum strain 3D7 was used to examine the potential of the cell microarray chip for malaria diagnosis. An erythrocyte suspension in a nuclear staining dye, SYTO 59, was dispersed on the chip surface, followed by 10 min standing to allow the erythrocytes to settle down into the microchambers. About 130 erythrocytes were accommodated in each microchamber, there being over 2,700,000 erythrocytes in total on a chip. A microarray scanner was employed to detect any fluorescence-positive erythrocytes within 5 min, and 0.0001% parasitemia could be detected. To examine the contamination by leukocytes of purified erythrocytes from human blood, 20 µl of whole blood was mixed with 10 ml of RPMI 1640, and the mixture was passed through a leukocyte isolation filter. The eluted portion was centrifuged at 1,000×g for 2 min, and the pellet was dispersed in 1.0 ml of medium. SYTO 59 was added to the erythrocyte suspension, followed by analysis on a cell microarray chip. Similar accommodation of cells in the microchambers was observed. The number of contaminating leukocytes was less than 1 on a cell microarray chip.
Conclusion: The potential of the cell microarray chip for the detection of malaria-infected erythrocytes was shown, it offering 10-100 times higher sensitivity than that of conventional light microscopy and easy operation in 15 min with purified erythrocytes.
Conflict of interest statement
Figures




References
-
- World Health Organization. World Health Organization; 2008. World Malaria Report 2008.pviii
-
- World Health Organization. World Health Organization; 2006. Guidelines for the treatment of malaria 2006. p. p8.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

