Chloroquine clinical failures in P. falciparum malaria are associated with mutant Pfmdr-1, not Pfcrt in Madagascar
- PMID: 20967251
- PMCID: PMC2954150
- DOI: 10.1371/journal.pone.0013281
Chloroquine clinical failures in P. falciparum malaria are associated with mutant Pfmdr-1, not Pfcrt in Madagascar
Abstract
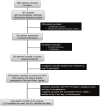
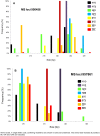
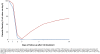
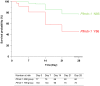
Molecular studies have demonstrated that mutations in the Plasmodium falciparum chloroquine resistance transporter gene (Pfcrt) play a major role in chloroquine resistance, while mutations in P. falciparum multidrug resistance gene (Pfmdr-1) act as modulator. In Madagascar, the high rate of chloroquine treatment failure (44%) appears disconnected from the overall level of in vitro CQ susceptibility (prevalence of CQ-resistant parasites <5%) or Pfcrt mutant isolates (<1%), strongly contrasting with sub-Saharan African countries. Previous studies showed a high frequency of Pfmdr-1 mutant parasites (>60% of isolates), but did not explore their association with P. falciparum chloroquine resistance. To document the association of Pfmdr-1 alleles with chloroquine resistance in Madagascar, 249 P. falciparum samples collected from patients enrolled in a chloroquine in vivo efficacy study were genotyped in Pfcrt/Pfmdr-1 genes as well as the estimation of the Pfmdr-1 copy number. Except 2 isolates, all samples displayed a wild-type Pfcrt allele without Pfmdr-1 amplification. Chloroquine treatment failures were significantly associated with Pfmdr-1 86Y mutant codon (OR = 4.6). The cumulative incidence of recurrence of patients carrying the Pfmdr-1 86Y mutation at day 0 (21 days) was shorter than patients carrying Pfmdr-1 86N wild type codon (28 days). In an independent set of 90 selected isolates, in vitro susceptibility to chloroquine was not associated with Pfmdr-1 polymorphisms. Analysis of two microsatellites flanking Pfmdr-1 allele showed that mutations occurred on multiple genetic backgrounds. In Madagascar, Pfmdr-1 polymorphism is associated with late chloroquine clinical failures and unrelated with in vitro susceptibility or Pfcrt genotype. These results highlight the limits of the current in vitro tests routinely used to monitor CQ drug resistance in this unique context. Gaining insight about the mechanisms that regulate polymorphism in Pfmdr1 remains important, particularly regarding the evolution and spread of Pfmdr-1 alleles in P. falciparum populations under changing drug pressure which may have important consequences in terms of antimalarial use management.
Conflict of interest statement
Figures
References
-
- Wootton JC, Feng X, Ferdig MT, Cooper RA, Mu J, et al. Genetic diversity and chloroquine selective sweeps in Plasmodium falciparum. Nature. 2002;418:320–323. - PubMed
-
- Snow RW, Trape JF, Marsh K. The past, present and future of childhood malaria mortality in Africa. Trends Parasitol. 2001;17:593–597. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

