Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning
- PMID: 20967758
- PMCID: PMC3025059
- DOI: 10.1002/hep.24006
Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning
Abstract
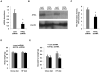
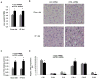
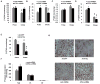
Despite advances in our understanding of the ways in which nutrient oversupply and triacylglycerol (TAG) anabolism contribute to hepatic steatosis, little is known about the lipases responsible for regulating hepatic TAG turnover. Recent studies have identified adipose triglyceride lipase (ATGL) as a major lipase in adipose tissue, although its role in the liver is largely unknown. Thus, we tested the contribution of ATGL to hepatic lipid metabolism and signaling. Adenovirus-mediated knockdown of hepatic ATGL resulted in steatosis in mice and decreased hydrolysis of TAG in primary hepatocyte cultures and in vitro assays. In addition to altering TAG hydrolysis, ATGL was shown to play a significant role in partitioning hydrolyzed fatty acids between metabolic pathways. Although ATGL gain and loss of function did not alter hepatic TAG secretion, fatty acid oxidation was increased by ATGL overexpression and decreased by ATGL knockdown. The effects on fatty acid oxidation coincided with decreased expression of peroxisome proliferator-activated receptor α (PPAR-α) and its target genes in mice with suppressed hepatic ATGL expression. However, PPAR-α agonism was unable to normalize the effects of ATGL knockdown on PPAR-α target gene expression, and this suggests that ATGL influences PPAR-α activity independently of ligand-induced activation.
Conclusion: Taken together, these data show that ATGL is a major hepatic TAG lipase that plays an integral role in fatty acid partitioning and signaling to control energy metabolism.
Copyright © 2010 American Association for the Study of Liver Diseases.
Conflict of interest statement
Figures


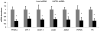

References
-
- Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, et al. Prevalence of hepatic steatosis in an urban population in the united states: Impact of ethnicity. Hepatology. 2004;40(6):1387–1395. - PubMed
-
- Watanabe S, Yaginuma R, Ikejima K, Miyazaki A. Liver diseases and metabolic syndrome. J Gastroenterol. 2008;43(7):509–518. - PubMed
-
- Lavine JE, Schwimmer JB. Nonalcoholic fatty liver disease in the pediatric population. Clin Liver Dis. 2004;8(3):549–58. - PubMed
-
- Jenkins CM, Mancuso DJ, Yan W, Sims HF, Gibson B, Gross RW. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J Biol Chem. 2004;279(47):48968–48975. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
