Age-related changes in mesenchymal stem cells derived from rhesus macaque bone marrow
- PMID: 20969724
- PMCID: PMC4339051
- DOI: 10.1111/j.1474-9726.2010.00646.x
Age-related changes in mesenchymal stem cells derived from rhesus macaque bone marrow
Abstract
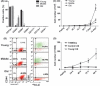
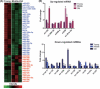
The regeneration potential of mesenchymal stem cells (MSCs) diminishes with advanced age and this diminished potential is associated with changes in cellular functions. This study compared MSCs isolated from the bone marrow of rhesus monkeys (rBMSCs) in three age groups: young (< 5 years), middle (8-10 years), and old (> 12 years). The effects of aging on stem cell properties and indicators of stem cell fitness such as proliferation, differentiation, circadian rhythms, stress response proteins, miRNA expression, and global histone modifications in rBMSCs were analyzed. rBMSCs demonstrated decreased capacities for proliferation and differentiation as a function of age. The production of heat shock protein 70 (HSP70) and heat shock factor 1 (HSF1) were also reduced with increasing age. The level of a core circadian protein, Rev-erb α, was significantly increased in rBMSCs from old animals. Furthermore, analysis of miRNA expression profiles revealed an up-regulation of mir-766 and mir-558 and a down-regulation of mir-let-7f, mir-125b, mir-222, mir-199-3p, mir-23a, and mir-221 in old rBMSCs compare to young rBMSCs. However, there were no significant age-related changes in the global histone modification profiles of the four histone core proteins: H2A, H2B, H3, and H4 on rBMSCs. These changes represent novel insights into the aging process and could have implications regarding the potential for autologous stem cells therapy in older patients.
Figures




References
-
- Auricchio A, Rivera VM, Clackson T, O'Connor EE, Maguire AM, Tolentino MJ, Bennett J, Wilson JM. Pharmacological regulation of protein expression from adeno-associated viral vectors in the eye. Mol. Ther. 2002;6:238–242. - PubMed
-
- Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22:675–682. - PubMed
-
- Beausejour C. Bone marrow-derived cells: the influence of aging and cellular senescence. Handb. Exp. Pharmacol. 2007;180:67–88. - PubMed
-
- Bellows CG, Pei W, Jia Y, Heersche JN. Proliferation, differentiation and self-renewal of osteoprogenitors in vertebral cell populations from aged and young female rats. Mech. Ageing Dev. 2003;124:747–757. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

