Postnatal development of depth-dependent collagen density in ovine articular cartilage
- PMID: 20969753
- PMCID: PMC2987790
- DOI: 10.1186/1471-213X-10-108
Postnatal development of depth-dependent collagen density in ovine articular cartilage
Abstract
Background: Articular cartilage (AC) is the layer of tissue that covers the articulating ends of the bones in diarthrodial joints. Adult AC is characterised by a depth-dependent composition and structure of the extracellular matrix that results in depth-dependent mechanical properties, important for the functions of adult AC. Collagen is the most abundant solid component and it affects the mechanical behaviour of AC. The current objective is to quantify the postnatal development of depth-dependent collagen density in sheep (Ovis aries) AC between birth and maturity. We use Fourier transform infra-red micro-spectroscopy to investigate collagen density in 48 sheep divided over ten sample points between birth (stillborn) and maturity (72 weeks). In each animal, we investigate six anatomical sites (caudal, distal and rostral locations at the medial and lateral side of the joint) in the distal metacarpus of a fore leg and a hind leg.
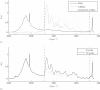
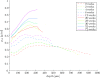
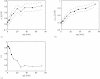
Results: Collagen density increases from birth to maturity up to our last sample point (72 weeks). Collagen density increases at the articular surface from 0.23 g/ml ± 0.06 g/ml (mean ± s.d., n = 48) at 0 weeks to 0.51 g/ml ± 0.10 g/ml (n = 46) at 72 weeks. Maximum collagen density in the deeper cartilage increases from 0.39 g/ml ± 0.08 g/ml (n = 48) at 0 weeks to 0.91 g/ml ± 0.13 g/ml (n = 46) at 72 weeks. Most collagen density profiles at 0 weeks (85%) show a valley, indicating a minimum, in collagen density near the articular surface. At 72 weeks, only 17% of the collagen density profiles show a valley in collagen density near the articular surface. The fraction of profiles with this valley stabilises at 36 weeks.
Conclusions: Collagen density in articular cartilage increases in postnatal life with depth-dependent variation, and does not stabilize up to 72 weeks, the last sample point in our study. We find strong evidence for a valley in collagen densities near the articular surface that is present in the youngest animals, but that has disappeared in the oldest animals. We discuss that the retardance valley (as seen with polarised light microscopy) in perinatal animals reflects a decrease in collagen density, as well as a decrease in collagen fibril anisotropy.
Figures



References
-
- Shapiro E, Borthakur A, Kaufman J, Leigh J, Reddy R. Water distribution patterns inside bovine articular cartilage as visualized by 1H magnetic resonance imaging. Osteoarthritis & Cartilage. 2001;9(6):533–538. - PubMed
-
- Benninghoff A. Form und Bau der Gelenkknorpel in ihren Beziehungen zur Funktion. Zweiter Teil: Der Aufbau des Gelenkknorpels in seinen Beziehungen zur Funktion. Zeitschrift für Zellforschung und Mikroskopische Anatomie. 1925;2:783–862. doi: 10.1007/BF00583443. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

