Posttranslational modification and regulation of glutamate-cysteine ligase by the α,β-unsaturated aldehyde 4-hydroxy-2-nonenal
- PMID: 20970495
- PMCID: PMC3014730
- DOI: 10.1016/j.freeradbiomed.2010.10.694
Posttranslational modification and regulation of glutamate-cysteine ligase by the α,β-unsaturated aldehyde 4-hydroxy-2-nonenal
Abstract
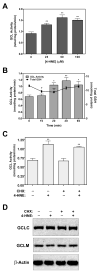
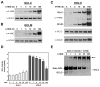
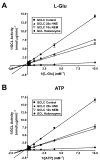
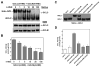
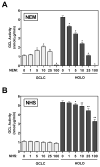
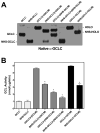
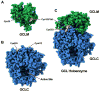
4-Hydroxy-2-nonenal (4-HNE) is a lipid peroxidation product formed during oxidative stress that can alter protein function via adduction of nucleophilic amino acid residues. 4-HNE detoxification occurs mainly via glutathione (GSH) conjugation and transporter-mediated efflux. This results in a net loss of cellular GSH, and restoration of GSH homeostasis requires de novo GSH biosynthesis. The rate-limiting step in GSH biosynthesis is catalyzed by glutamate-cysteine ligase (GCL), a heterodimeric holoenzyme composed of a catalytic (GCLC) and a modulatory (GCLM) subunit. The relative levels of the GCL subunits are a major determinant of cellular GSH biosynthetic capacity and 4-HNE induces the expression of both GCL subunits. In this study, we demonstrate that 4-HNE can alter GCL holoenzyme formation and activity via direct posttranslational modification of the GCL subunits in vitro. 4-HNE directly modified Cys553 of GCLC and Cys35 of GCLM in vitro, which significantly increased monomeric GCLC enzymatic activity, but reduced GCL holoenzyme activity and formation of the GCL holoenzyme complex. In silico molecular modeling studies also indicate these residues are likely to be functionally relevant. Within a cellular context, this novel posttranslational regulation of GCL activity could significantly affect cellular GSH homeostasis and GSH-dependent detoxification during periods of oxidative stress.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures

References
-
- Ando Y, Brannstrom T, Uchida K, Nyhlin N, Nasman B, Suhr O, Yamashita T, Olsson T, El Salhy M, Uchino M, Ando M. Histochemical detection of 4-hydroxynonenal protein in Alzheimer amyloid. J Neurol Sci. 1998;156:172–176. - PubMed
-
- Carbone DL, Doorn JA, Kiebler Z, Ickes BR, Petersen DR. Modification of heat shock protein 90 by 4-hydroxynonenal in a rat model of chronic alcoholic liver disease. J Pharmacol Exp Ther. 2005;315:8–15. - PubMed
-
- Rahman I, van Schadewijk AA, Crowther AJ, Hiemstra PS, Stolk J, MacNee W, De Boer WI. 4-Hydroxy-2-nonenal, a specific lipid peroxidation product, is elevated in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:490–495. - PubMed
-
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. - PubMed
-
- Sayre LM, Lin D, Yuan Q, Zhu X, Tang X. Protein adducts generated from products of lipid oxidation: focus on HNE and one. Drug Metab Rev. 2006;38:651–675. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

